Translate this page into:
Use of Thromboelastography Platelet Mapping for Assessment of Individual Platelet Response Secondary to Oral Antiplatelet Therapy after Percutaneous Coronary Intervention: An Attempt to Start Personalized Antiplatelet Therapy in India
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
High on-treatment platelet reactivity (HPR) with P2Y12 receptor antagonists in patients treated with dual antiplatelet therapy (DAPT) is strongly associated with adverse ischemic events after percutaneous coronary intervention (PCI). This prospective study was conducted to assess individual platelet response and HPR to antiplatelet medications in post-PCI cases by thromboelastography platelet mapping (TEG-PM). Total 82 patients who were on aspirin and on either clopidogrel, prasugrel, or ticagrelor were evaluated. The percentage of platelet inhibition to arachidonic acid (AA) and adenosine diphosphate (ADP) was calculated by [100-{(MA ADP/AA–MA Fibrin) / (MA Thrombin–MA Fibrin) × 100}], taking 50% response as cut-off for HPR. HPR to clopidogrel and prasugrel was 14.29 and 12.5%, respectively. No HPR was detected to aspirin and ticagrelor. The mean percentage of platelet inhibition was significantly higher in patients with ticagrelor 82.99, 95% confidence interval (CI) of [77.3, 88.7] as compared with clopidogrel 72.21, 95% CI of [65.3, 79.1] and prasugrel 64.2, 95% CI of [52.5, 75.9] (p-value of 0.041 and 0.003, respectively). Aspirin along with ticagrelor is associated with a higher mean percentage of platelet inhibition, and lower HPR as compared with the usage of aspirin combined with clopidogrel or prasugrel. Additionally, it might also be concluded that TEG-PM could be used effectively to measure the individual platelet functions which would make oral antiplatelet therapy more personalized for cardiac patients.
Keywords
thromboelastography
platelet mapping
high on-treatment platelet reactivity
antiplatelet therapy
personalized medicine
Introduction
Platelet aggregation is considered the most important factor in the development of ischemic complications after percutaneous coronary intervention (PCI).1 Dual antiplatelet therapy (DAPT) with acetylsalicylic acid and a P2Y12 receptor antagonist reduces the risk of thrombosis after PCI in acute coronary syndrome cases.2 Although, clopidogrel is the commonest P2Y12 receptor antagonist used as an oral antiplatelet agent in combination with aspirin; still a significant percentage of patients experience thrombotic events due to inadequate response to clopidogrel therapy.3 The poor responsiveness to clopidogrel leads to a high on-treatment platelet reactivity (HPR) to adenosine diphosphate (ADP) which is a recognized risk factor for post-PCI thrombotic events.4 Clopidogrel requires metabolism via the CYP (cytochrome P450) pathway to an active metabolite. It is well-established that patients carrying CYP2C19 loss-of-function alleles have a reduced capacity for clopidogrel bioactivation, impaired platelet inhibition, and a significantly higher risk of thrombotic complications compared with patients without a CYP mutation.5 Next-generation P2Y12 receptor antagonists, like prasugrel or ticagrelor, are developed to overcome this limitation of clopidogrel as CYP2C19 genotype that does not impact their clinical effectiveness.6, 7 Although CYP2C19 genotype-based strategy to guide P2Y12 inhibitor selection after PCI is a routine clinical practice in many developed countries and especially recommended in populations with Asian ancestry8 but it is a distant dream in country like India because of the cost of pharmacogenomic tests and lack of adequate resources. Rather individual platelet function assessment could be considered as an alternative strategy to this pharmacogenomic approach in developing countries to identify HPR in patients. Light transmittance platelet aggregometry is considered as “gold-standard assay” for platelet function test (PFT) but other methods, like VerifyNow, thromboelastography platelet mapping (TEG-PM), multiple platelet function analyzer (Multiplate), platelet function assay (PFA)-100, vasodilator-stimulated phosphoprotein (VASP) flowcytometry, and PlateletWorks,9 could also be used effectively as point-of-care PFTs. TEG-PM is a modified PFT which is used to look specifically at functional platelet inhibition secondary to antiplatelet therapy10 and has been shown to correlate well with the light transmittance platelet aggregometry11 to quantify the effect of antiplatelet medications.
The primary objective of this study was to assess the extent of HPR to clopidogrel, prasugrel, and ticagrelor by TEG-PM in a group of patients who underwent PCI due to coronary artery disease. Secondary objective was to identify which P2Y12 receptor antagonist displays higher percentage of platelet inhibition without increasing the bleeding risks in the same group.
Materials and Methods
Study Details
This was a prospective, observational, single-center cohort study where TEG-PM was performed in a group of patients to assess the platelet functions after starting DAPT. All these patients underwent PCI due to coronary artery disease (CAD) from June 2016 to December 2018 at a cardiac center in Eastern India. Institutional review board (ECR/587/Inst/WB/2014/RR-17) approval was obtained for this study. Informed consent was taken from each patient before PCI.
Study Population and Design
Inclusion criteria for this study were patients aged more than 18 years, had confirmed CAD, and underwent PCI. All patients considered for the study were on aspirin and on either clopidogrel, prasugrel, or ticagrelor in combination. The exclusion criteria were known cases of bleeding diathesis, had a history of drug allergy against any of the antiplatelet drug, a platelet count less than 100 × 109/L or greater than 500 × 109/L, history of active bleeding within the previous 3 months, any other major surgical procedure within 2 weeks prior to the PCI, known case of active liver disease, prothrombin time >1.5 times of control, hematocrit <30%, creatinine >0.354 mmol/L, any recent use of nonsteroidal anti-inflammatory drugs (NSAIDs), or glycoprotein (GP) IIb/IIIa inhibitors. During the study period, 105 patients initially met the inclusion criteria. Among 105 patients, 23 were excluded from this study because they had gastrointestinal reflux after starting of DAPT maintenance dose, leading to change of medications or premature stoppage of one antiplatelet drug or history of irregular drug intake. Finally, a total of 82 patients were tested by TEG-PM assay and data were collected prospectively. On the day of PCI procedure, a loading dose of 325-mg aspirin was administered in each patient along with a loading dose of either 300-mg clopidogrel, 60-mg prasugrel, or 180-mg ticagrelor. Maintenance dose of P2Y12 receptor antagonist (clopidogrel, 75-mg once per day; prasugrel, 10-mg once per day; or ticagrelor, 90-mg twice daily) was started after 24 hours of procedure along with maintenance dose of aspirin (75–100 mg once per day). The response to the treatment was assessed in each patient by TEG-PM after 1 week of starting DAPT. Patients with HPR to a specific P2Y12 receptor antagonist were switched over to another combination of DAPT and followed-up clinically.
Pharmacology of P2Y12 Receptor Antagonists
Clopidogrel and prasugrel are thienopyridine prodrugs that transformed into active metabolites by hepatic CYP enzymes. Those active metabolites bind to the platelets, P2Y12 receptors are irreversible and produce inhibition of platelet function for the lifetime of the affected platelet. Most of the clopidogrel is hydrolyzed by carboxylesterase-1, leaving only trace amount available for active metabolite formation, which occurs predominantly by CYP2C19.5 Prasugrel is dependent to a lesser extent on CYP2C19 metabolism to form its active metabolite.6 Ticagrelor is a directly acting cyclopentyltriazolopyrimidine class molecule which is a reversible, noncompetitive P2Y12 receptor antagonist and does not require metabolism by CYP2C19.7 Clopidogrel has a slow onset of action, whereas ticagrelor provides the fastest platelets inhibition.
Blood Sampling and Platelet Function Analysis
Blood samples were obtained from patients in a fasting state during their first follow-up visit at cardiology outpatient department (OPD) after 1 week of starting DAPT. Samples were collected into separate vacutainer blood collecting tubes (BD Vacutainer, Becton Dickinson, United States) containing 3.2% trisodium citrate or lithium heparin. After discarding the first 2 to 3 mL of blood, the Vacutainer tubes were filled to capacity and gently inverted 3 to 5 times to ensure complete mixing of the anticoagulant. Blood samples were analyzed in the transfusion medicine laboratory by trained personnel within an hour of receiving the blood sample as per the manufacturer instructions.10 Both analyzer (series 5000) and the reagents were from Haemoscope Corporation (Niles, Illinois, United States). The following TEG parameters were studied: reaction time (R), time to initial fibrin formation up to 2 mm; K time (K), time to clot formation up to 20 mm; α angle (α), speed of clot formation; and maximum amplitude (MA), measurement of clot strength. The normal reference values of TEG parameters (as provided by the manufacturer) for kaolin-activated citrated samples were taken as R = 2 to 8 minutes, K = 1 minute to 3 minutes, α = 55 to 78 degrees, and MA = 51 to 69 mm. Heparin was used as an anticoagulant to eliminate thrombin activity in the sample during assay. Reptilase and activated factor XIII (activator F) were used to generate a cross-linked fibrin clot to isolate the fibrin contribution in clot strength. For maximal clot strength (MA Thrombin) 1 mm of citrate stabilized blood was transferred to a vial containing kaolin and mixed by gentle inversion. Kaolin activated blood (340 μL) was added to a TEG cup containing 20 μL of 0.2 M CaCl2. As MA, which is representing the maximal clot strength, can be ascertained by the binding of activated platelets to a fibrin mesh, 360 μL of heparinized blood was added to 10 μL of activator F (reptilase and factor XIIIa) and the contribution of each fibrin meshwork to clot strength (MA Fibrin) was assessed. The contribution of P2Y12 receptor or cyclooxygenase pathways to the clot formation can be measured by the addition of the appropriate agonist, ADP, or arachidonic acid (AA). Therefore, heparinized blood (360 μL) was added to a TEG cup in the presence of the activator F and agonist, 10 μ: ADP (2 μM, final concentration), yielding the (MA ADP) or 10 μL AA (1 μM, final concentration) for the MA AA. The percentage of platelet inhibition to agonist was calculated by TEG-PM software as [100-{(MA ADP/AA–MA Fibrin) / (MA Thrombin–MA Fibrin) X 100}].
Assessment of Outcome
The percentage of HPR to clopidogrel, prasugrel, and ticagrelor was assessed and considered as the primary outcome of the study. The cut-off for HPR was set at 50% in response to the individual drug similar to the cut-off followed in platelet aggregometry. The percentage of platelet inhibition to each P2Y12 receptor antagonist was also assessed during this study.
Statistical Analysis
Data were collected and analyzed by using Microsoft Excel statistics software package. Continuous variables were expressed as mean with standard deviation and categorical data were expressed as frequencies or percentages. Student’s t-test was applied for comparison of means and significance was established with p-values level of 0.05 (p < 0.05).
Results
All the demographic variables and baseline data were found similar in studied population (Table 1). The mean age of patients was 63.5 years (range: 44.5–73 years) and the mean weight was 58.3 kg (range: 47–81 kg). All patients were male. All 82 patients were treated with aspirin, 42 among them were treated with clopidogrel in combination, 16 with prasugrel, and 24 with ticagrelor in combination, respectively (Table 2). Fig. 1 is showing the number of HPR cases to each P2Y12 receptor antagonist. Table 2 and Fig. 2 are showing that the mean percentage of platelet inhibition was significantly higher in patients with ticagrelor 82.99, 95% confidence interval (CI) of [77.3, 88.7] as compared with clopidogrel 72.21, 95% CI of [65.3, 79.1] and prasugrel 64.2, 95% CI of [52.5, 75.9] (p-value of 0.041 and 0.003, respectively). No bleeding manifestation was observed in any patient due to administration of DAPT.
|
Mean ± standard deviation |
Range |
|
|---|---|---|
|
Abbreviations: AA, arachidonic acid; MA, maximal amplitude; R, K and α are standard thromboelastography parameters. |
||
|
Age (y) |
63.5 ± 4.22 |
44.5–73 |
|
Body weight (kg) |
58.3 ± 4.6 |
47–81 |
|
Baseline R (min) |
4.17 ± 0.96 |
2–7.3 |
|
Baseline K (min) |
1.8 ± 0.29 |
1.05–2.95 |
|
Baseline α (degrees) |
64.17 ± 3.98 |
55.2–74.6 |
|
Baseline MA (MACK in mm) |
61.9 ± 2.71 |
55.5–68 |
|
AA (MAAA in mm) |
16.3 ± 5.73 |
4.3–29.2 |
|
Platelets inhibition% with AA |
80.69 ± 9.14 |
61.1–100 |
|
Clopidogrel |
Prasugrel |
Ticagrelor |
|
|---|---|---|---|
|
Abbreviation: HPR: High on-treatment platelet reactivity. |
|||
|
Patients tested |
42 |
16 |
24 |
|
Patients with HPR |
6 |
2 |
0 |
|
HPR (%) |
14.29 |
12.50 |
0 |
|
Mean platelet inhibition (%) |
72.21 |
64.20 |
82.99 |
|
Standard deviation |
22.85 |
23.80 |
14.19 |
|
Standard error of means |
3.52 |
5.95 |
2.89 |
|
p-Value of means |
0.041 |
0.003 |
– |
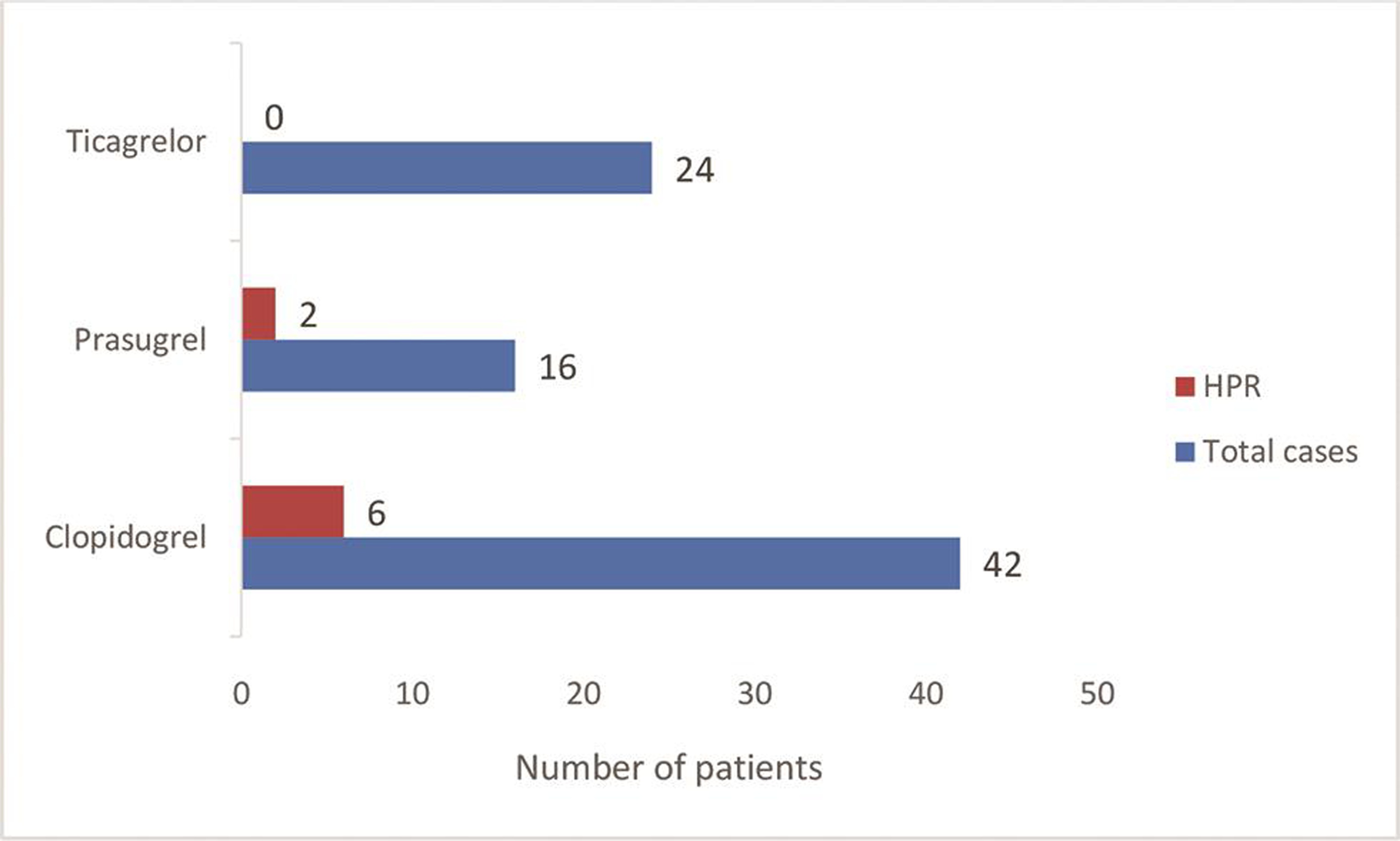
- Number of HPR cases in patients treated with antiplatelet drugs. HPR, high on-treatment platelet reactivity.

- Mean platelet inhibition (%) due to antiplatelet drugs.
Discussion
Numerous studies have demonstrated substantial variability in platelet inhibition among patients treated with clopidogrel depending on the method of PFT and the definition of drug response.12 In our study, TEG-PM analysis was used to measure the HPR and it was found 14.29% to clopidogrel which is in agreement with other global studies on clopidogrel response where platelet response was evaluated either by optical light transmission platelets aggregometry or by flowcytometry.13, 14, 15, 16 The P2Y12 receptor antagonist used in a post-PCI case is chosen based on the clinical assessment of risk for bleeding and cost of the therapy. Although, large randomized controlled trials like ARCTIC trial (the assessment by a double randomization of a conventional antiplatelet strategy versus a monitoring-guided strategy for drug-eluting stent implantation and of treatment interruption vs. continuation of 1 year after stenting) or GRAVITAS trial (the gauging responsiveness with a VerifyNow assay—impact on thrombosis and safety) failed to demonstrate a sustainable clinical benefit of using a PFT-guided approach to antiplatelet therapy17, 18 but the investigators of these studies have used PFT-guided dose escalation of clopidogrel rather than switch over to other DAPT combination. It is a well-known fact that in patients with CYP2C19 mutations, even maintenance doses as high as 300 mg daily of clopidogrel did not result in adequate platelet inhibition.19 No HPR to ticagrelor was found in our study which is almost similar to the results of Dalal et al.20 Although HPR to prasugrel has not been well reported, it was found 12.5% in our study which is in agreement with the results of Bonello et al21 where VASP method was used for assessment of platelet response. It was previously reported that ticagrelor has a mean percentage platelet inhibition of 80 to 90% when tested by platelets aggregometry22 which is again consistent with the current study finding of a mean platelet inhibition of 82.99% with ticagrelor. Average percentage of platelet inhibition in patients treated with prasugrel (64.2%) was lower than clopidogrel (72.21%) in our study which is in agreement with the TRILOGY-ACS trial (targeted platelet inhibition to clarify the optimal strategy to medically manage acute coronary syndromes) where prasugrel was not found superior to clopidogrel in patients who had acute coronary syndromes (ACS).23 Although, meta-analysis24 of several studies showed a stronger antiplatelet effect of ticagrelor over prasugrel which is in favor of the present study; the recent ISAR-REACT 5 trial (intracoronary stenting and antithrombotic regimen 5)25 showed that a stronger antiplatelet effect is not always associated with better composite end results in ACS cases. There was no bleeding manifestation with ticagrelor therapy despite higher platelet inhibition which is similar to the results of the PLATO trial (platelet inhibition and patient outcomes).26 Also, no HPR to aspirin was found in our study which is in agreement with the results of Tantry et al.27 There were no ischemic complications observed after 6-month follow-up in patients with HPR who were switched over to another DAPT combination depending on TEG-PM results.
Limitations
The major limitation of our study is the nonrandomized design. The sample size is small because of the poor affordability to this relatively costly test. Also, the follow-up test after switch over to a new DAPT combination was not possible due to the increase cost of the assay. Lack of gender distribution of results could be considered as another limitation as only male patients were evaluated. We did not have any provision to compare our test results with a more sensitive testing platforms like VerifyNow which has a defined HPR value for P2Y12 reaction assessment. Furthermore, the pharmacokinetic analysis of drug levels in blood was not performed in this study. Hence, precise correlation with the observed effects of the drugs is not possible.
Conclusion
Although, measurements of platelet reactivity can vary over time in a significant proportion of patients and treatment adjustment according to a single PFT at a single time point might not be sufficient for guiding antiplatelet therapy, but it is nearly impossible to use “gold-standard” technologies like CYP2C19 genotyping, platelets aggregometry, or flowcytometry routinely to monitor the effect of antiplatelet therapy in resource-limited health care facilities. Therefore, we conclude that aspirin along with ticagrelor is associated with a higher mean percentage of platelet inhibition and lower HPR as compared with the usage of aspirin with clopidogrel or prasugrel. In addition to that, it might also be concluded that TEG-PM could be used effectively to measure the individual platelet functions in response to antiplatelet drugs and the decision of switching to another combination of DAPT in HPR cases could be taken based on this assessment. This treatment approach might be useful to make oral antiplatelet therapy more personalized for cardiac patients in developing countries.
Acknowledgment
We are thankful to all the staffs of department of transfusion medicine for their support.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
Ethical approval was obtained from the Institution Ethics Committee of The Mission Hospital (ECR/587/Inst/WB/2014/RR-17) for this study.
Consent
Individual informed written consent was obtained from each patient before PCI.
Data Transparency
Raw data are available; data sheet can be shared on request.
Authors’ Contributions
S.S.D. and N.A.M. designed the study; S.S.D. performed the tests; collected data and prepared the draft. D.D. provided support during data analysis. S.S.D. prepared the manuscript. All authors reviewed the manuscript before submission.
Funding We did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors for this study.
References
- Platelet activation in myocardial ischemic syndromes. Expert Rev Cardiovasc Ther. 2004;2(4):535-545.
- [Google Scholar]
- Medical management after coronary stent implantation: a review. JAMA. 2013;310(2):189-198.
- [Google Scholar]
- Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107(23):2908-2913.
- [Google Scholar]
- Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Cardiol. 2005;46(10):1820-1826.
- [Google Scholar]
- Clopidogrel pharmacogenomics and risk of inadequate platelet inhibition: US FDA recommendations. Pharmacogenomics. 2009;10(11):1799-1817.
- [Google Scholar]
- Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119(19):2553-2560.
- [Google Scholar]
- PLATO investigators. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376(97/49):1320-1328.
- [Google Scholar]
- CYP2C19 genotype has a greater effect on adverse cardiovascular outcomes following percutaneous coronary intervention and in Asian populations treated with clopidogrel: a meta-analysis. Circ Cardiovasc Genet. 2014;7(6):895-902.
- [Google Scholar]
- Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010;303(8):754-762.
- [Google Scholar]
- TEG 5000 haemostasis analyser: User manual. Available at: https://studylib.net/doc/18643089/pn06-510-teg-5000-user-manual. Accessed January 18, 2020
- Quantifying the effect of antiplatelet therapy: a comparison of the platelet function analyzer (PFA-100) and modified thromboelastography (mTEG) with light transmission platelet aggregometry. Anesthesiology. 2006;105(4):676-683.
- [Google Scholar]
- Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate? J Am Coll Cardiol. 2007;49(6):657-666.
- [Google Scholar]
- Individual variations of platelet inhibition after loading doses of clopidogrel. J Intern Med. 2002;252(3):233-238.
- [Google Scholar]
- Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb Haemost. 2003;89(5):783-787.
- [Google Scholar]
- Dual antiplatelet drug resistance in patients with acute coronary syndrome. Indian Heart J. 2009;61(1):68-73.
- [Google Scholar]
- Identifica-tion of low responders to a 300-mg clopidogrel loading dose in patients undergoing coronary stenting. Thromb Res. 2005;115(1/02):101-108.
- [Google Scholar]
- ARCTIC Investigators. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367(22):2100-2109.
- [Google Scholar]
- GRAVITAS Investigators. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305(11):1097-1105.
- [Google Scholar]
- Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA. 2011;306(20):2221-2228.
- [Google Scholar]
- Oral antiplatelet therapy and platelet inhibition: An experience from a tertiary care center. Indian Heart J. 2016;68(5):624-631.
- [Google Scholar]
- High on-treatment platelet reactivity after prasugrel loading dose and cardiovascular events after percutaneous coronary intervention in acute coronary syndromes. J Am Coll Cardiol. 2011;58(5):467-473.
- [Google Scholar]
- Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120(25):2577-2585.
- [Google Scholar]
- TRILOGY ACS Investigators. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367(14):1297-1309.
- [Google Scholar]
- High on-treatment platelet reactivity with ticagrelor versus prasugrel: a systematic review and meta-analysis. J Thromb Haemost. 2015;13(6):931-942.
- [Google Scholar]
- Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019;381(16):1524-1534.
- [Google Scholar]
- PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057.
- [Google Scholar]
- Overestimation of platelet aspirin resistance detection by thrombelastograph platelet mapping and validation by conventional aggregometry using arachidonic acid stimulation. J Am Coll Cardiol. 2005;46(9):1705-1709.
- [Google Scholar]







