Translate this page into:
Role of Cerebral Oximetry in Extracorporeal Membrane Oxygenation
Naman Shastri, MD Department of Cardiac Anesthesia, Visiting Faculty for Academics, Training and Research, U.N. Mehta Institute of Cardiology and Research Center, Ahmedabad, Gujarat, India; Department of Anesthesia, Shri Sathya Sai Institute of Higher Medical Science Bangalore, Karnataka 380016 India nashastri@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cerebral oximetry, which is based on near-infrared spectroscopy (NIRS) technology, is an optical technique that allows for noninvasive and continuous monitoring of brain oxygenation by determining cerebral tissue blood oxygen saturation. Many research and observational studies were performed with neonates using various types of NIRS/cerebral oximetry monitors. However, no food and drug administration (FDA) approved-cerebral oximeter is available for neonates. Successful validation of cerebral oximetry for the FDA has been done in human adult volunteer studies under protocols in which jugular bulb and arterial blood samples were obtained under different levels of fractional inspired oxygen levels.
Keywords
ARDS
cerebral oximetry
extracorporeal membrane oxygenation
Introduction
To secure hemodynamic stability, extracorporeal membrane oxygenation (ECMO) is one treatment modality for temporary bridging in this critical early postoperative phase of acute respiratory distress syndrome (ARDS) patients. As maintenance of adequate tissue oxygenation is essential in critical ill patients, goal-directed hemodynamic optimization targeting a mixed venous oxygen saturation (SvO2) greater than 70% has been shown to reduce postoperative organ dysfunction and length of hospital stay in patients after cardiac Surgery.1 As very recent publications have clearly shown that central venous oxygen saturation cannot be used as a substitute for SvO2 in this setting of ECMO, the use of a pulmonary artery catheter (PAC) for determination of SvO2 may still be regarded as a “gold standard.” However, the insertion of a PAC in patients undergoing venoarterial ECMO, that is, right atrial cannulation, is technically demanding. Additionally, the prolonged use of a PAC is not without risk. Thus, a noninvasive but continuous technique would be desirable for the early detection of deterioration of the global oxygen balance. One such technique is near-infrared spectroscopy (NIRS). Cerebral oximetry was first described more than 25 years ago and has recently been investigated in the context. Cerebral oximeters calculate cerebral oxygenation using NIRS technology based on a modified light absorbent theory called the Beer-Lambert law. According to the Beer-Lambert law, an amount of a substance or compound, in this case, oxygen, can be determined by how much light the substance absorbs.
Cerebral oximetry, which is based on NIRS technology, is an optical technique that allows for noninvasive and continuous monitoring of brain oxygenation by determining cerebral tissue blood oxygen saturation (SctO2). Many research and observational studies were performed with neonates using various types of NIRS/cerebral oximetry monitors.2, 3, 4 However, no food and drug administration (FDA)-approved cerebral oximeter is available for neonates. Successful validation of cerebral oximetry for the FDA has been done in human adult volunteer studies under protocols in which jugular bulb and arterial blood samples were obtained under different levels of fractional inspired oxygen (FiO2) levels.5, 6, 7
Cerebral Oximetry for Neonates during Extracorporeal Membrane Oxygenation
At the initiation of extracorporeal life support, a decision is first made as to whether the infant would best be served with venovenous (VV) or venoarterial (VA) support. More than 60% of neonatal ECMO patients reported in the Extracorporeal Life Support Organization (ELSO) registry have received treatment with VA bypass.8 In neonates with respiratory failure, VA-ECMO is gradually being replaced by a VV technique, which uses a single double-lumen catheter.
The catheter is placed in the right atrium, where blood is drained and reinfused into the same chamber, thus requiring cannulation of only the right jugular vein, sparing the carotid artery. Other advantages of VV-ECMO include maintenance of normal pulsatile blood flow and the theoretical advantage that particles entering the ECMO circuit enter by way of the pulmonary rather than the systemic circulation.
Research by Raïs-Bahrami et al resulted in the development of a new catheter design that significantly lowers the degree of recirculation.9 This catheter design in 12, 15, and 18 Fr. sizes allows the use of VV-ECMO in a greater number of infants. In VV-ECMO, the proximal end of internal jugular vein is also cannulated for cephalad drainage. This catheter is placed at the jugular bulb to augment cerebral venous outflow. The jugular bulb catheter is connected to the venous tubing of the ECMO circuit via a Luer connector with a three-way stopcock to facilitate blood sampling for cephalic venous saturation measurement. This catheter allows access for brain venous blood needed to validate neonatal cerebral oximetry in a routine clinical setting.
Cerebral Oximetry Monitoring in Neonates While on Extracorporeal Membrane Oxygenation
Cerebral oximetry is based on NIRS techniques where light is emitted at one point and sensed by a detector at a second point after passage through a medium such as biological tissue.10 A cerebral oximetry sensor is placed on the forehead, and if there is adequate space, off to one side of the forehead to monitor one brain hemisphere as shown in Fig. 1A. Bilateral left and right forehead sensors could also be used if collateral flow in the brain hemispheres is a concern. Bilateral left and right forehead sensors could also be used if collateral flow in the brain hemispheres is a concern. In healthy subjects, bilateral sensor measurements are similar. Light from the sensor passes through the scalp and skull to interrogate primarily the gray matter of brain tissue as shown in Fig. 1B. As a result of the high light scattering properties of biological tissue, a small amount of light reaches the sensor detector and can be used for spectroscopic analysis. Interrogation depth of the sensor is estimated to be of light source to detector separation distance and also depends on scalp and skull thickness that must be subtracted from the sensor's overall interrogation depth.11 Light source to detector separation is of 25 mm. For example, for a 1-month-old infant that has an estimated scalp thickness of 3 mm 27 and a skull thickness of 4 mm, the resultant estimated brain tissue interrogation depth for the sensor is about 6 mm (Fig. 2).

- (A–C) Cerebral oximetry sensor.
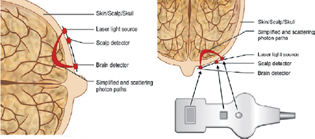
- Cerebral oximetry sampling of tissue from two photodetectors.
Therefore, SctO2 values are usually below arterial oxygen saturation (i.e., [SaO2] or pulse oximeter arterial oxygen saturation [SpO2]) values and above brain venous oxygen saturation (i.e., cephalad catheter internal jugular oxygen saturation) values. Unlike pulse oximetry, cerebral oximetry does not need pulsatile flow to make a measurement. Therefore, cerebral oximetry offers a means to monitor brain blood oxygenation during circulatory arrest or cardiopulmonary bypass utilizing nonpulsatile flow, whereas a pulse oximeter is nonfunctional. When used in conjunction with pulse oximetry, cerebral oximetry offers a promising method to determine noninvasively SvO2 representative of cephalad or jugular bulb oxygen saturation under many physiological states when peripheral pulse oximetry SpO2 is representative of brain SaO2 and the venous to arterial blood volume ratio of 70 to 30% as described previously holds true.
SctO2 is much more sensitive to brain oxygenation compared to pulse oximetry. For neonates, pulse oximetry SpO2 readings typically vary from 85 to 100%. Even when SpO2 is high (95–100%), SctO2 can vary from 30 to 100%. This fluctuation is dependent on oxygen supply and demand, which can be affected by cerebral metabolism and hypercapnia. Trend cerebral oxygenation monitoring focuses more on the amount of change from an established baseline cerebral oxygenation value, whereas absolute cerebral oxygenation monitoring focuses more on the meaning of the cerebral oxygenation value. For example, a decrease in cerebral oxygenation from 80 to 70% might be of concern for trend monitoring because there was a drop in cerebral oxygenation by 10%. However, if monitored by an absolute cerebral oximeter, a change in cerebral oxygenation by 10% may not be of concern if an established reference range for this patient is 65 to 80%.
Issues and Validation with Cerebral Oximetry during Extracorporeal Membrane Oxygenation
One concern regarding accuracy of adult cerebral oximetry is the issue of signal contamination by the extracranial tissue layers. Compared to adults, neonates have a much thinner skull and scalp thickness, which theoretically result in less extracranial interference. Another concern regarding accuracy is comparison of cerebral oximetry parameters to a gold standard. For cerebral oximetry validation in adults, SctO2 is compared to the weighted global measures of 70% SvO2 from jugular bulb oximetry and 30% SaO2 under controlled conditions based on relative venous to arterial blood volume in the microvasculature. The lower accuracy of cerebral oximetry in neonate applications could be attributable to an immature neonatal cerebral circulatory system, resulting in a more variable venous to arterial blood volume ratio from the average 70:30 used to validate cerebral oximetry. Other possibilities include increased contamination of cephalad brain venous blood with extracerebral tissues, possibly due to the small vessel diameters in relation to the sampling catheter that could result in nonideal placement of the catheter. SctO2 is a relativity new parameter. Lack of experience in the use of the parameter can therefore confuse the clinical interpretation of the measurements. SctO2 values are below SaO2 and above venous oxygen saturation values because of the mixed arterial–venous blood in the microvasculature. Clinicians are familiar with measuring the arterial to venous oxygen saturation difference to infer oxygen consumption in tissue.12, 13, 14
Conclusion
The cerebral oximeter measurements agree well with the measured cerebral venous saturation. This noninvasive method of measuring cerebral tissue and venous saturation could easily be recommended as a substitute for drawing venous blood samples in order to measure cerebral venous saturations in neonates requiring extracorporeal life support.
Conflict of Interest
None declared.
References
- Temporary extracorporeal membrane oxygenation in patients with refractory postoperative cardiogenic shock–a single center experience. J Card Surg. 2003;18(6):512-518.
- [Google Scholar]
- Cotside measurement of cerebral blood flow in ill newborn infants by near infrared spectroscopy. Lancet. 1988;2:770-771. (8614):
- [Google Scholar]
- Cerebral oxygen monitoring with near infrared spectroscopy: clinical application to neonates. J Clin Monit. 1991;7(4):325-334.
- [Google Scholar]
- Fetal and neonatal cerebral oxygen monitoring with NIRS: theory and practice. Early Hum Dev. 1992;29:269-273. (1-3):
- [Google Scholar]
- Impact of hypoxemia on the performance of cerebral oximeter in volunteer subjects. J Neurosurg Anesthesiol. 2000;12(3):201-209.
- [Google Scholar]
- Validation in volunteers of a near-infrared spectroscope for monitoring brain oxygenation in vivo. Anesth Analg. 1996;82(2):269-277.
- [Google Scholar]
- Using the CAS cerebral oximeter to estimate cerebral venous oxygen saturation. Anesthesiology. 2005, as a abstracts.com; A164
- [Google Scholar]
- Ann Arbor, MI, July 2005
- Improved oxygenation with reduced recirculation during venovenous extracorporeal membrane oxygenation: evaluation of a test catheter. Crit Care Med. 1995;23(10):1722-1725.
- [Google Scholar]
- Non-invasive neuroimaging using near-infrared light. Biol Psychiatry. 2002;52(7):679-693.
- [Google Scholar]
- Thickness of the normal skull in the American Blacks and Whites. Am J Phys Anthropol. 1975;43(1):23-30.
- [Google Scholar]
- Using the CAS cerebral oximeter to estimate cerebral venous oxygen saturation. Anesthesiology. 2005, as a abstracts.com; A16
- [Google Scholar]
- Validation of the CAS adult cerebral oximeter during hypoxia in healthy volunteers. IARS 80th Clinical and Scientific Congress. Anesth Analg. 2006;102:S-162.
- [Google Scholar]






