Translate this page into:
suPAR as a risk Prediction Biomarker in Extracorporeal Membrane Oxygenation
*Corresponding author: Mohit Prakash, Senior Resident, Department of Cardiac Anaesthesiology and Critical Care, Cardiac Thoracic Centre, All India Institute of Medical Sciences, New Delhi, India. mohitprakashdx@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Prakash M, Mujahid OM, Singh R. suPAR as a risk prediction biomarker in extracorporeal membrane oxygenation. J Card Crit Care TSS 2023;7:65-70.
Abstract
suPAR is a promising biomarker of cardiovascular diseases, as it reflects “low-grade inflammation” and is associated with lifestyle factors such as smoking, alcohol, and an inactive lifestyle. suPAR is expressed in various cells involved in the development of atherosclerosis, including macrophages, endothelial cells, and smooth muscle cells, and an accumulation of suPAR in the atheroma has also been found. suPAR plays a role in the coagulation cascade during plasminogen activation and fibrinolysis. This abstract provides description of three case series showing the utility of suPAR as a risk prediction biomarker on VA extracorporeal membrane oxygenation (ECMO). We used SUPAR in 15 patients undergoing cardiac surgery of which three went on VA ECMO. Herein, we describe in detail three such patients and discuss each with good review of the literature.
Keywords
suPAR
VA ECMO
Biomarker
Risk prediction
INTRODUCTION: WHAT IS suPAR?
The soluble urokinase plasminogen activator receptor (suPAR) level is elevated in patients with cardiovascular diseases compared to healthy individuals, and elevated suPAR level is associated with: either a atherosclerosis,[1] a ischemic heart disease (IHD),[2-4] a poor prognosis,[2,5-7] or a venous thromboembolism.[8]
suPAR is a promising biomarker of cardiovascular diseases, as it reflects “low-grade inflammation” and is associated with lifestyle factors such as smoking, alcohol, and an inactive lifestyle. The previous studies have shown, that the urokinase plasminogen activator/urokinase plasminogen activated receptor system (uPA/uPAR-system) plays a key role in the pathogenesis of atherosclerosis.[1] Physiologically, the system is involved in fibrinolysis, angiogenesis, and immune function, including leukocyte migration, proliferation, and degradation of matrix during tissue remodeling in the atherosclerotic plaque.[2]
suPAR is expressed in various cells involved in the development of atherosclerosis, including macrophages, endothelial cells, and smooth muscle cells, and an accumulation of suPAR in the atheroma has also been found.[9] suPAR plays a role in the coagulation cascade during plasminogen activation and fibrinolysis.[10] However, no causal relationship between the urokinase system and atherosclerosis or cardiovascular diseases has been shown.[2] However, new data have demonstrated an association between suPAR and focal segmental glomerulosclerosis. The kidneys play a key role in blood pressure and fluid balance regulation, and therefore, suPAR may be associated with heart failure and myocardial strain.[11,12] A similar suPAR-mediated effect on endothelial cells and platelets may potentially play a role in vascular inflammation and thrombosis.[11] We describe with three case references, the utility of suPAR as a risk prediction biomarker on VA extracorporeal membrane oxygenation (ECMO). We used SUPAR in 15 patients undergoing cardiac surgery of which three went on VA ECMO. Herein, we describe in detail three such patients and discuss each with good review of the literature.
CASE SERIES
Case 1
A 34-year-old female with hemorrhagic fever was having a low PO2, for which a VA ECMO was initiated of her dengue fever when she was not responding to conventional treatment. The patient remained stable, as a case of “single organ failure” with a temperature around 37°C. On day 18 on VA ECMO, there was a new development with bleeding from NJ, NG at cannula sites. There was no new requirement for inotrope and her platelets were 96 × 10/L with an INR of 1.6 and fibrinogen of 2.6 g/L.
Problems faced in this patient were as follows: For her ongoing bleeding; should tranexamic acid be given? What else needs investigating as urea and creatinine seemed to increase on day 4 of VA ECMO inserted for low cardiac output (CO) Tranexamic would be appropriate as the TEG was consistent with primary fibrinolysis [Figure 1]. We investigated the circuit which showed high clot burden in the circuit, plasma free hemoglobin, and raised post oxygenator PaO2, with hemolysis oliguria. Decided to do suPAR levels to rule out sepsis and judge the renal status in detail as well.
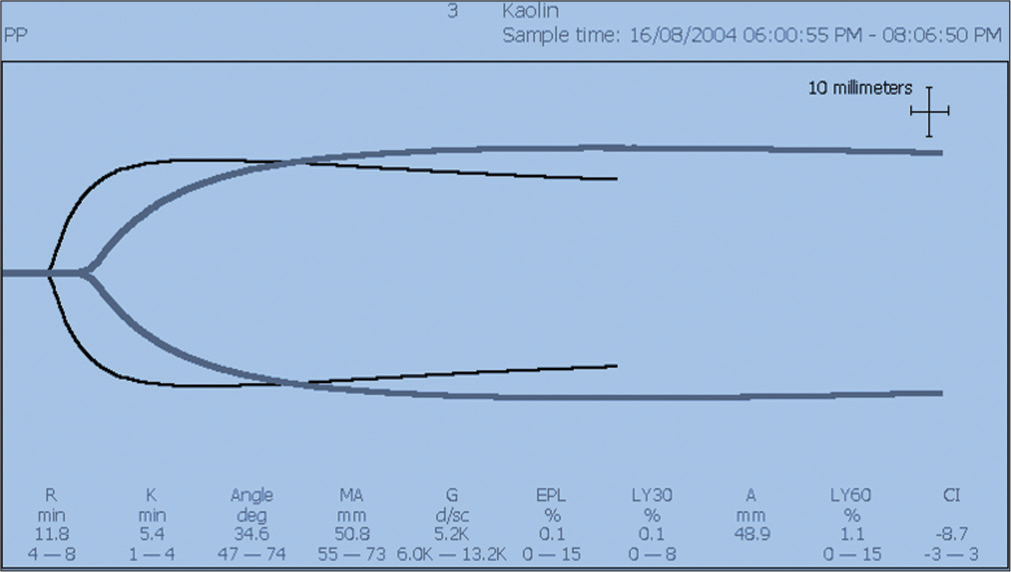
- Classic TEG showing primary fibrinolysis on this patient of dengue fever with fibrinolysis.
Bleeding onset patient previously stable, now as per guidelines, we considered it as a case of sepsis and DIC requiring no antifibrinolytic therapy. Circuit failure and fibrinolysis were managed with TEG guidance [Figure 1].
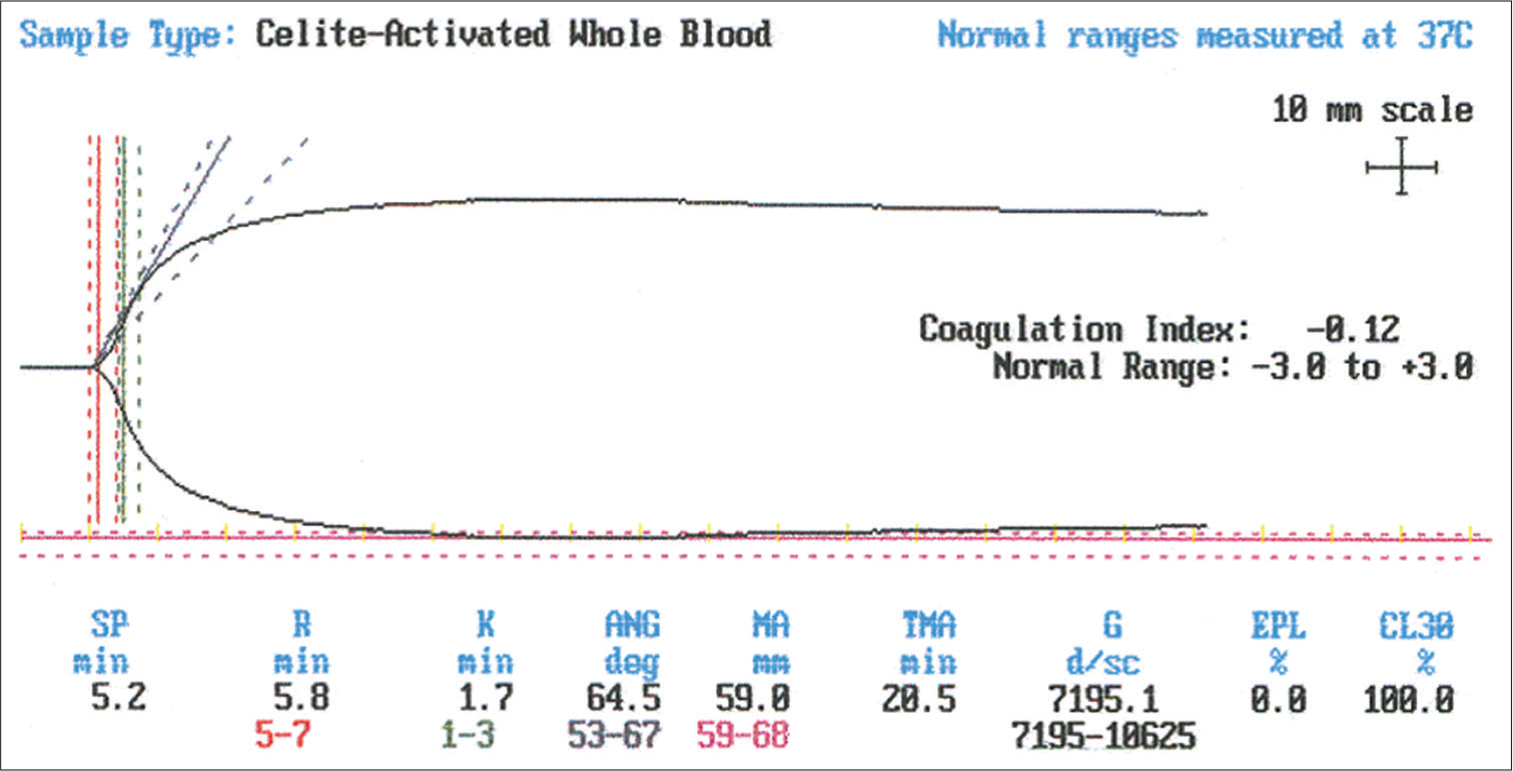
- Normal TEG with no fibrinolysis.
It is important to rule out thrombus, for which a detailed ECHO and Doppler done on this patient did not show any thrombus or vegetation. ELSO registry states that thrombosis necessitating circuit change occurs in 20% of VA ECMO patients with a high incidence of occult thromboembolic events in autopsy studies (>70%). There is increased risk of thrombosis with increased duration of ECMO. Vigilance following timely weaning and decannulation is important too (limb ischemia or compromise). A slow CVVH was then added to the VA ECMO on Day 20 and by Day 22 the patient was settled and with stable hemodynamic and RFT’s. Timely, CVVH or slow ultrafiltration added to ECMO takes care of rising RF values and timely dialysis. The high urea and creatinine were the culprits for raised suPAR levels. suPAR levels on day 22 were 14 and remained between 10 and 14 till day 48. Once ECMO was weaned off, suPAR came down as creatinine levels came down on day 48–1.2. A repetitive TEG was done on her [Figures 1 and 2], which was useful in guiding the right blood product on this septic patient on VA ECMO. Cardiac output (CO) and PO2 improved on day 16. The suPAR levels were 8 ng/mL on day 6 and on day 28 with ongoing CVVH and judicious cryoprecipitate and platelet rich plasma (PRP) it reduced to 4 ng/mL. Sepsis was avented with tazoboctam and meropenem timely intravenously.
Discussion
In uremic patients receiving peritoneal dialysis or hemodialysis or slow CVVH, the suPAR level and the carotid intima-media thickness (IMT) in the two dialysis groups are significantly higher than in healthy age- and sex-matched controls, and in a smaller study, suPAR is also associated with IMT.[13]
suPAR is also a prognostic marker of cardiovascular diseases in patients with mild-to-moderate chronic renal diseases, including cardiac mortality, non-fatal MI, myocardial ischemia, coronary intervention, ischemic stroke, and newly diagnosed peripheral vascular diseases.[3] suPAR and eGFR are comparable in estimating mortality risk; however, in this population, suPAR was a stronger cardiovascular risk predictor than eGFR.[3]
Lyngbaek et al.[5] observed that in patients admitted with suspected acute coronary syndrome (ACS), suPAR is a strong predictor of mortality and of readmission due to heart failure and a new myocardial infarction. Thus, in non-survivors, the baseline suPAR level was significantly higher than in survivors, and, similarly, the suPAR level was higher in readmitted patients than in non-readmitted patients. The authors concluded that in patients with suspected ACS, suPAR improves risk stratification beyond traditional risk factors.[5]
In a South African study, the baseline suPAR level was not able to predict development of hypertension, but, on the other hand, a change in suPAR level was to some extent associated with increasing blood pressure during the observation period.[14] In our study too, suPAR was raised in dengue patient with mild AKI.
Case 2
In a Tetralogy of Fallot, 2-year’s-old male, child with a intracardiac repair done on hypothermia, at 6 h post-CPB after protamine neutralization, VA ECMO initiated due to low CO (1.1 L/min). This patient had a cardiac arrest, while VA central ECMO was being initiated. Temperature was 34°C–pH was warmed, with an initial VA ECMO flow of 4 L/min and gradually reduced to 2.5–2.8 L/min.
At 6 h, a SUPAR level reads at 9.002 ng/mL which was critically high. The patient went into ventricular tachycardia, a suPAR level at 10 h post-DC shock for VT reads at 11.08 ng/mL. Post-hemodynamics shock, of 10J and 15J (internal DC shock), and sinus rhythm reverted with xylocard and magnesium. Low flow of 1.5–2 L/min of VA ECMO continued, with adequate drainage and arterial blood gases all within normal limits. At this point, suPAR at 36 h on VA ECMO came down to 4.2 and then at 60 h to 3.8 ng/mL. Subsequently, on day 8, VA ECMO was weaned off and the patient was stable, maintaining good CO (CI–2.84 L/min ICON).
Discussion
In patients resuscitated from a cardiac arrest and treated with hypothermia, suPAR was studied as a potential prognostic tool. One study found that the suPAR level 6 h after the cardiac arrest was strongly associated with mortality and neurological outcome.[6] Similarly, in another study, suPAR was strongly associated with mortality but not with neurological outcome.[7] In patients with non-shockable rhythms, the baseline suPAR level was significantly higher than in patients with shockable rhythms.[7]
High suPAR levels are associated with incidence of venous thromboembolism.[8] No significant association between pulmonary embolism and suPAR was found in the literature so far.[8] In males and females with carotid plaques, the suPAR level is significantly higher than in individuals with no carotid plaques.[4,15] Similarly, suPAR is a predictor of IHD, and in patients with both elevated suPAR level and carotid plaques, the risk of developing IHD is significantly increased.[4] Following adjustment for traditional risk factors and subclinical organ damage, suPAR remains associated with cardiovascular mortality.[15]
Case 3
Patient vital stats
Post Bentall 30-year-male patient continues to drain for 7 h, no effect of any blood product. Hemodynamics are low CO and unstable BP with ECG findings of inferior lead ischemia is present. The suPAR, here, is 28.02 ng/mL. The patient initiated on peripheral VA ECMO on day 4 of ICU stay. Post Bentall, the heparin infusion is topped as ROTEM tests done, shows, as described in [Figure 3]. The RCA graft is revised as a redo second surgery next day and patient put on VA ECMO and IABP, both on day 4.A case of prolonged CT in Intem >240 s showing heparin influence with decreased platelet function. After giving Protamine, Intem CT is normal which confirms bleeding was due to heparin [Figure 4]. Hence, the patient on VA ECMO with no heparin for 3 days had suPAR levels down to 4.0 ng/ mL and all bleeding stopped and post-RCA re-implantation in a saplienae and venous graft. The patient did well till day 32 of discharge with 15 days value if suPAR showing high suPAR levels on VA ECMO. Hence, the suPAR level at discharge was 1.8 ng/ mL levels. The suPAR levels should be taken as risk predictor of morbidity/mortality outcomes, while on ECMO!
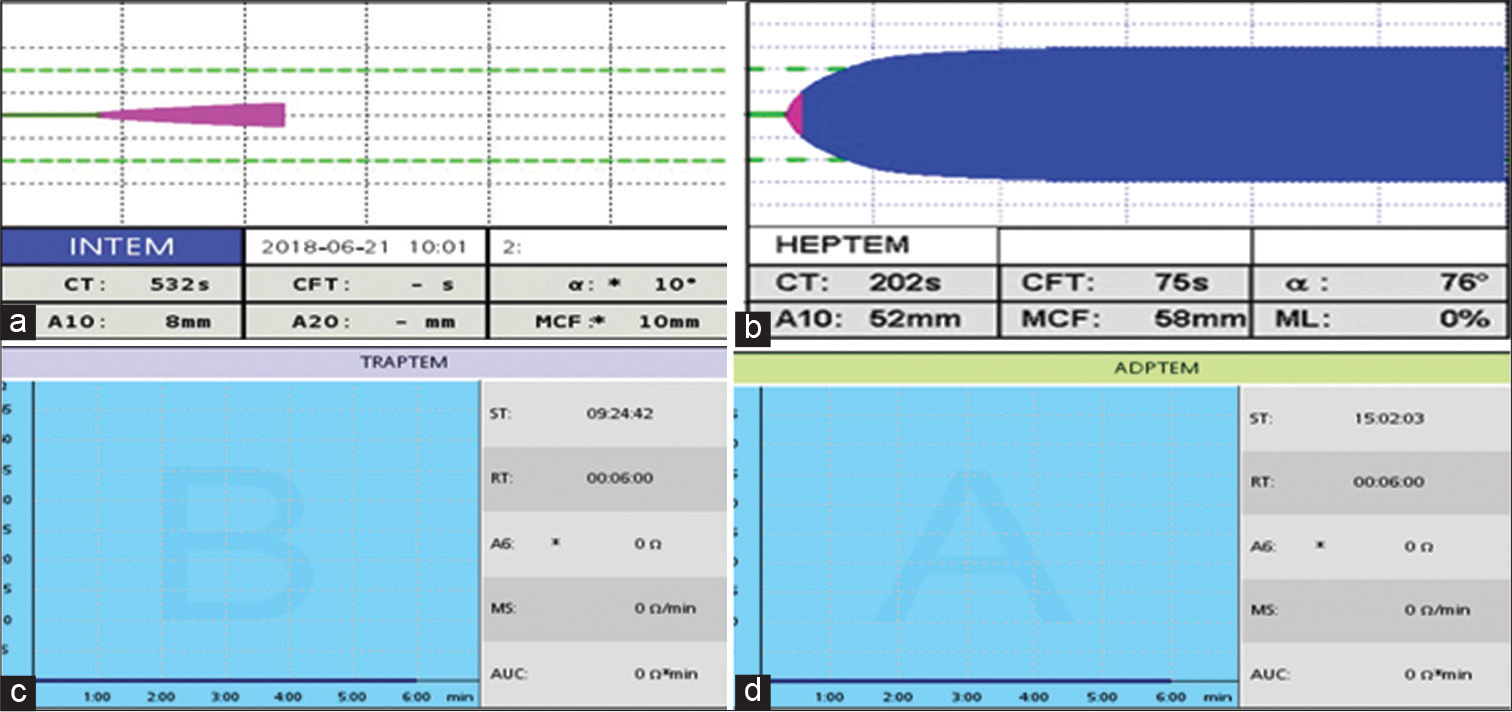
- (a and b): Intem and Heptem values showing prolonged CT imparting prolonged heparin influence and (c and d): Markedly reduced platelet function with TRAPTEM and ADPTEM reagents on ROTEM.
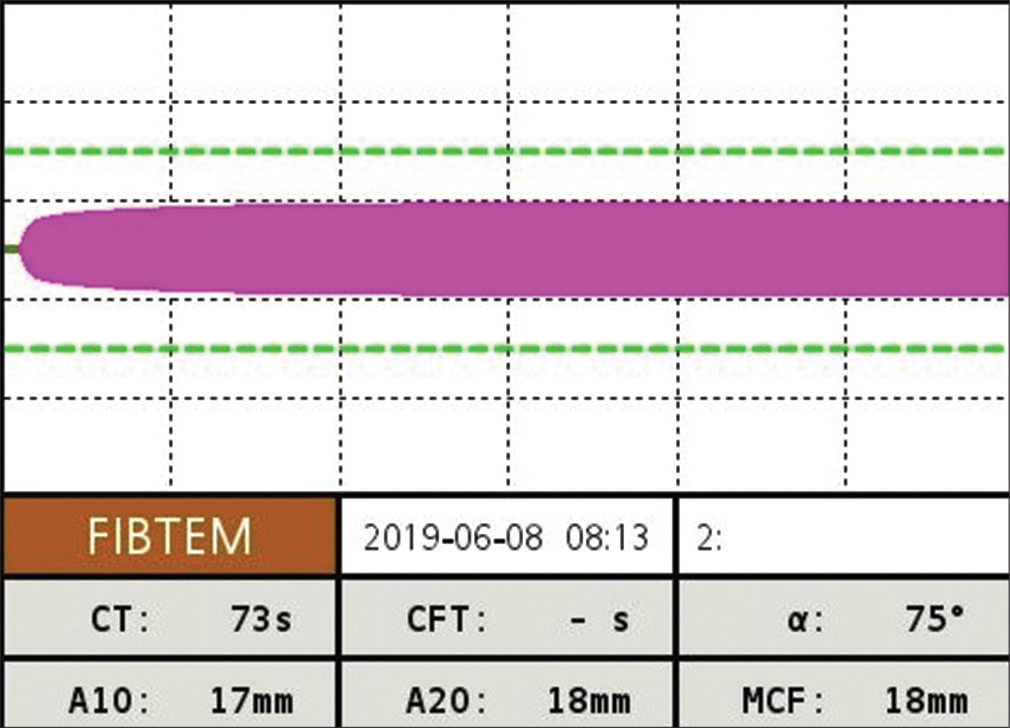
- Post-protamine FIBTEM levels of ROTEM are normal.
Discussion
In five large studies, including a total of 4866 individuals,[16-19] suPAR is a predictor of cardiovascular morbidity and mortality in the general population even after adjustment for the well-validated Framingham risk score,[19] and taking into account well-known risk factors, C-reactive protein (CRP), and other biomarkers associated with cardiovascular diseases. In general, the prognostic value of baseline suPAR level appears to be strongest in the younger age groups and in males.[19]
Review of literature of suPAR over the years
In patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention, the suPAR level is elevated the first 24 h after admission. Following adjustment for traditional risk factors, age, sex, CRP, creatinine, troponin T, total cholesterol, diabetes, and hypertension, suPAR remains associated with mortality and a new myocardial infarction.[2] In non-survivors, baseline suPAR values are significantly higher than in survivors (4.9 ng/mL vs. 3.9 ng/mL), and all-cause mortality increases significantly with higher suPAR values.[2] Following adjustment for sex and age, suPAR was associated with an increased risk of developing atrial fibrillation. However, this association disappeared following adjustment for well-known risk factors.[12]
Biomarkers are critical instruments in terms of risk prediction. Emerging data proves that new pathways and pathophysiological hypotheses yield biomarkers beyond the established ones.[20-24] The failure of suPAR in the diagnosis of AMI seems surprising given the results of previous smaller studies.[5,25] Both studies did not report the diagnostic value of suPAR, but rather it has a prognostic value in AMI patients. With a median 3.8 ng/mL in AMI patients and 3.3 ng/mL in patients with non-AMI, suPAR levels seem in range with current references.[26,27] Nevertheless, the two previous reports in patients with ACS report clearly higher values.[5,25] The early Danish report from 2013 in 449 consecutive chest pain patients in a single-center yielded a median suPAR level of 5.80 ng/mL for fatal AMI, and 4.44 for non-fatal AMI using the same assay for suPAR measurement as in our study. In line with this finding, median maximum hs-TnT level in the Danish study was 149 ng/L. Similarly, in a recent Austrian case-cohort study (n = 194), serum suPAR levels were 29% higher in the 57 AMI (NSTEMI) patients (our study: 13%), compared with the 76 healthy controls. Again, hs-Tn levels were relatively high, at a mean of 986.3 ng/L in AMI, and the rate of subjects diagnosed with AMI was 74% compared with only 20% in our study.
Feroza et al. measured suPAR in 318 patients who underwent cardiac positron emission tomography for the evaluation of ischemia. Patients were followed for outcomes (median follow-up time 1.91 years) including death and major adverse cardiovascular events (MACE). They used linear regression to assess the association between log-transformed suPAR levels and ischemia as estimated by summed stress score (SSS), myocardial blood reserve (MFR), and myocardial blood flow (MBF) adjusting for cardiovascular risk factors. Authors used Cox proportional hazards to assess the association between suPAR, death and MACE. Area under the curves were used to characterize the predictive value of suPAR and stress testing in predicting outcomes. The cohort consisted of 191 men (60.1%), which had a mean age of 61.6 ± SD 11.82 years. The median suPAR value 4.17 pg/mL (3.73–4.61). One hundred and thirty-seven patients (43.7%) had a SSS score of ≥10. They noted a modest correlation between suPAR and MBF (0.32, P < 0.001) and MFR (−0.37, P < 0.001). suPAR was independently associated with MBF and MFR. At a median follow-up of 1.91 years, there were 35 deaths and 79 MACE events. suPAR levels of >4.09 were independently associated with death (HR 18.28, 95% CI [4.252–78.566]) and MACE (HR 3.245, 95% CI [1.749–6.020]) after adjusting for comorbidities.
The uPAR acts as receptor for urokinase (uPA) that itself splits inactive plasminogen into active plasmin.[28] Beyond this function, by interaction with other proteins, uPAR also plays a role in important atherosclerotic cell processes such as migration, adhesion, angiogenesis, proliferation, and chemotaxis.[29,30] The circulating biomarker suPAR is the soluble form of the cell membrane-bound protein uPAR.[31] It is produced by cleavage of membrane-bound uPAR and can be detected in plasma and serum.[32] The suPAR blood level is stable with no diurnal variation and no changes following fasting.[33] It is discussed as a new marker for immune activation and inflammation – pathways, which are important in atherogenesis.[34]
CONCLUSION
suPAR is an immune-derived signaling protein with serum levels reflecting chronic inflammation. SuPAR levels are strongly predictive of cardiovascular outcomes in various patient populations and outperform other biomarkers. Whether suPAR levels correlate with ischemia severity or MBF and MFR is unknown. We sought to assess the relationship between suPAR levels and coronary blood flow and determine thresholds for predicting outcomes. Taken together, one may hypothesize, that our cohort of 15 patients represents a relative low-risk cohort, and that incremental value of suPAR on top of hs-Tn might only evolve in midand high-risk cohorts. Our results suggest that suPAR is associated with ischemia at the microcirculatory level and can help in risk stratification of patients being evaluated for coronary disease, while the patients remain on ECMO.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- The urokinase system in the pathogenesis of atherosclerosis. Atherosclerosis. 2012;222:8-14.
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness of soluble urokinase plasminogen activator receptor to predict repeat myocardial infarction and mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous intervention. Am J Cardiol. 2012;110:1756-63.
- [CrossRef] [PubMed] [Google Scholar]
- Soluble urokinase receptor is a biomarker of cardiovascular disease in chronic kidney disease. Kidney Int. 2015;87:210-6.
- [CrossRef] [PubMed] [Google Scholar]
- Soluble urokinase plasminogen activator receptor: A risk factor for carotid plaque, stroke, and coronary artery disease. Stroke. 2014;45:18-23.
- [CrossRef] [PubMed] [Google Scholar]
- Soluble urokinase plasminogen activator receptor for risk prediction in patients admitted with acute chest pain. Clin Chem. 2013;59:1621-9.
- [CrossRef] [PubMed] [Google Scholar]
- The inflammatory marker suPAR after cardiac arrest. Ther Hypothermia Temp Manag. 2015;5:89-94.
- [CrossRef] [PubMed] [Google Scholar]
- The predictive value of soluble urokinase plasminogen activator receptor (SuPAR) regarding 90-day mortality and 12-month neurological outcome in critically ill patients after out-of-hospital cardiac arrest. Data from the prospective FINNRESUSCI study. Resuscitation. 2014;85:1562-7.
- [CrossRef] [PubMed] [Google Scholar]
- Soluble urokinase plasminogen activator receptor and incidence of venous thromboembolism. Thromb Haemost. 2015;115:657-62.
- [CrossRef] [PubMed] [Google Scholar]
- Soluble urokinase plasminogen activator receptor is associated with inflammation in the vulnerable human atherosclerotic plaque. Stroke. 2012;43:3305-12.
- [CrossRef] [PubMed] [Google Scholar]
- The urokinase receptor: Focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett. 2010;584:1923-30.
- [CrossRef] [PubMed] [Google Scholar]
- Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916-25.
- [CrossRef] [PubMed] [Google Scholar]
- Increased plasma level of soluble urokinase plasminogen activator receptor is associated with incidence of heart failure but not atrial fibrillation. Eur J Heart Fail. 2014;16:377-83.
- [CrossRef] [PubMed] [Google Scholar]
- The urokinase-type plasminogen activator/its soluble receptor system is independently related to carotid atherosclerosis and associated with CC-chemokines in uraemic patients. Thromb Res. 2008;122:328-35.
- [CrossRef] [PubMed] [Google Scholar]
- Soluble urokinase plasminogen activator receptor and hypertension among black South Africans after 5 years. Hypertens Res. 2015;38:439-44.
- [CrossRef] [PubMed] [Google Scholar]
- Soluble urokinase plasminogen activator receptor is associated with subclinical organ damage and cardiovascular events. Atherosclerosis. 2011;216:237-43.
- [CrossRef] [PubMed] [Google Scholar]
- Soluble urokinase plasminogen activator receptor as a prognostic marker of all-cause and cardiovascular mortality in a black population. Int J Cardiol. 2015;184:631-6.
- [CrossRef] [PubMed] [Google Scholar]
- Soluble urokinase plasminogen activator receptor is in contrast to high-sensitive C-reactive-protein associated with coronary artery calcifications in healthy middle-aged subjects. Atherosclerosis. 2014;237:60-6.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268:296-308.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular risk prediction in the general population with use of suPAR, CRP, and Framingham Risk Score. Int J Cardiol. 2013;167:2904-11.
- [CrossRef] [PubMed] [Google Scholar]
- Testosterone levels and Type 2 diabetes-no correlation with age, differential predictive value in men and women. Biomolecules. 2018;8:76.
- [CrossRef] [PubMed] [Google Scholar]
- Novel DNA methylation sites influence GPR15 expression in relation to smoking. Biomolecules. 2018;8:74.
- [CrossRef] [PubMed] [Google Scholar]
- Intrinsic iron release is associated with lower mortality in patients with stable coronary artery disease-first report on the prospective relevance of intrinsic iron release. Biomolecules. 2018;8:72.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse outcome prediction of iron deficiency in patients with acute coronary syndrome. Biomolecules. 2018;8:60.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of iron-homeostasis regulating peptide hepcidin in coronary heart disease-evidence from the large atherogene study. Biomolecules. 2018;8:43.
- [CrossRef] [PubMed] [Google Scholar]
- Multibiomarker analysis in patients with acute myocardial infarction. Eur J Clin Investig. 2017;47:638-48.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative analysis of novel cardiovascular biomarkers in patients with chronic heart failure. Eur J Intern Med. 2017;44:31-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pathway-specific aggregate biomarker risk score is associated with burden of coronary artery disease and predicts near-term risk of myocardial infarction and death. Circ Cardiovasc Qual Outcomes. 2017;10:e001493.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of urokinase plasminogen activator (uPA)-mediated atherosclerosis: Role of the uPA receptor and S100A8/A9 proteins. J Biol Chem. 2011;286:22665-77.
- [CrossRef] [PubMed] [Google Scholar]
- Activated human neutrophils rapidly release the chemotactically active D2D3 form of the urokinase-type plasminogen activator receptor (uPAR/CD87) Mol Cell Biochem. 2009;321:111-22.
- [CrossRef] [PubMed] [Google Scholar]
- Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J Biol Chem. 1991;266:1926-33.
- [CrossRef] [PubMed] [Google Scholar]
- Complexes between urokinase-type plasminogen activator and its receptor in blood as determined by enzyme-linked immunosorbent assay. Int J Cancer. 1998;77:236-42.
- [CrossRef] [Google Scholar]
- Soluble urokinase plasminogen activator receptor is a marker of dysmetabolism in HIV-infected patients receiving highly active antiretroviral therapy. J Med Virol. 2008;80:209-16.
- [CrossRef] [PubMed] [Google Scholar]
- Inflammation and plaque vulnerability. J Intern Med. 2015;278:483-93.
- [CrossRef] [PubMed] [Google Scholar]






