Translate this page into:
Preoperative Combined Adiposity–Nutritional Index Predicts Major aDverse Cardiac and Cerebral Events following Off-pump coRonary Artery Revascularization (PANDORA): A Retrospective Single-Center Study
Dr. ItiShri, MD Department of Cardiac Anaesthesia, Atal Bihari Vajpayee Institute of Medical Sciences (ABVIMS) and Dr. Ram Manohar Lohia Hospital Baba Kharak Singh Marg, New Delhi 110001 India iti.anesthesia@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background The metabolic–nutritional profile of coronary artery disease (CAD) patients can be an important outcome determinant. A high visceral adiposity index (VAI) and a low prognostic nutritional index (PNI) have been described to predict major adverse cardiac and cerebral events (MACCE) in nonoperative settings and poor cardiac-surgical outcomes, respectively. The present study evaluated the MACCE-predictive value of the two indices, in isolation and as a combined adiposity–nutritional index (CANI = VAI/PNI) in patients undergoing off-pump coronary artery bypass grafting (OPCABG).
Methods The retrospective study was conducted in 1207 OPCABG patients at a tertiary cardiac care center. Thirty-day postoperative data was evaluated for the development of MACCE, defined by any of the following: cardiac arrest, ST-segment elevation myocardial ischemia (STEMI), repeat coronary revascularization, or stroke. The perioperative characteristics of the MACCE and no-MACCE groups were analyzed for the predictors of postoperative MACCE.
Results One-hundred thirty-two patients (10.93%) developed MACCE postoperatively. On univariate analysis, age, EuroSCORE II, ejection fraction, diabetes mellitus, asymptomatic carotid artery disease, left main (LM) disease, PNI, and VAI predicted MACCE. Subsequent to multivariate analysis, age, EuroSCORE II, and CANI were the independent predictors. The MACCE predictive cutoffs of VAI, PNI, and CANI were 3.2, 38.46, and 0.075 (area under the curve [AUC]; sensitivity; specificity: 0.64; 77%; 81.3%, 0.77; 92.6%, 65%, 0.78; 64.5%; 80.2%, respectively). CANI correlated positively with duration of mechanical ventilation, length of intensive care unit (ICU) stay, and mean postoperative vasoactive inotropic scores (VIS). CANI ≥ 0.075 was also associated with a higher incidence of postoperative atrial fibrillation, low cardiac output syndrome, and acute kidney injury.
Conclusions CANI emerged as an independent predictor of MACCE following OPCABG.
Keywords
coronary revascularization
major adverse cardiac and cerebral events (MACCE)
off-pump coronary artery bypass grafting
postoperative outcomes
prognostic nutritional index
visceral adiposity index
Introduction
While coronary artery disease (CAD) in itself predisposes to the occurrence of major adverse cardiac and cerebral events (MACCE), the involved risk is further accentuated in perioperative settings.1 Needless to say, a gamut of factors can potentially predispose to postoperative MACCE,2, 3, 4 albeit the risk attributable to the preoperative metabolic and nutritional status has received little research attention. This becomes particularly relevant in cardiac surgical settings given the fact that the perioperative cardiac surgical conduct entails a heightened risk of MACCE.1, 2, 3
Appropriate to the context, indices such as visceral adiposity index (VAI)5, 6 and prognostic nutritional index (PNI)7, 8, 9 can serve as important surrogates of the metabolic and nutritional risk profile. On one end, independent research groups suggest a superior prognostic significance of VAI over its individual components (body mass index [BMI], high-density lipoprotein (HDL), triglyceride (TG), waist circumference [WC]).5, 10 On the other end, PNI takes immunonutritive parameters like hypoalbuminemia and lymphopenia into account that can be of perioperative importance, in particular.9, 11 While an elevated VAI has been described to be associated with an increased incidence of MACCE in nonoperative CAD settings,5 the description of poor cardiac surgical outcomes in background of a reduced PNI is equally noteworthy.11 In addition, a recent study by Engin et al outlines a high preoperative VAI and a low PNI as risk factors for atrial fibrillation (AF) following coronary artery bypass grafting (CABG).10
However, in the most holistic sense of the matter, the cardiometabolic risk profiling is closely interlinked with the nutritional predisposition to poor surgical outcomes. Considering the proposition that studying the composite metabolic–nutritional risk can be of incremental value, we sought to retrospectively investigate the MACCE predictive value of VAI and PNI in isolation and as a combined adiposity–nutritional index (CANI = VAI/PNI) in patients undergoing off-pump CABG (OPCABG).
Materials and Methods
After obtaining clearance from the Institutional Ethics Committee (No.601 (36/2020) IEC/ABVIMS/RMLH), the data of 1,412 patients who underwent elective OPCABG from January 2015 to December 2020 at our tertiary care referral center was retrospectively evaluated. The patients who underwent emergency surgery, those with preexisting neurological deficit and stroke, recent myocardial infarction (MI) within 4 preoperative weeks, preoperative arrhythmias, any active infection, and critically ill patients (patients on mechanical ventilation, intra-aortic balloon pump (IABP), and/or inotropes), were excluded from the study. The patients who had to be converted to on-pump CABG intraoperatively were excluded from the study. The absence of laboratory data within 3 preoperative days was also considered as a study exclusion. After accounting for the loss to follow-up, the data of 1207 patients were finally compiled from the hospital archives and electronic database. The flowchart for patient enrollment is illustrated in Fig. 1.
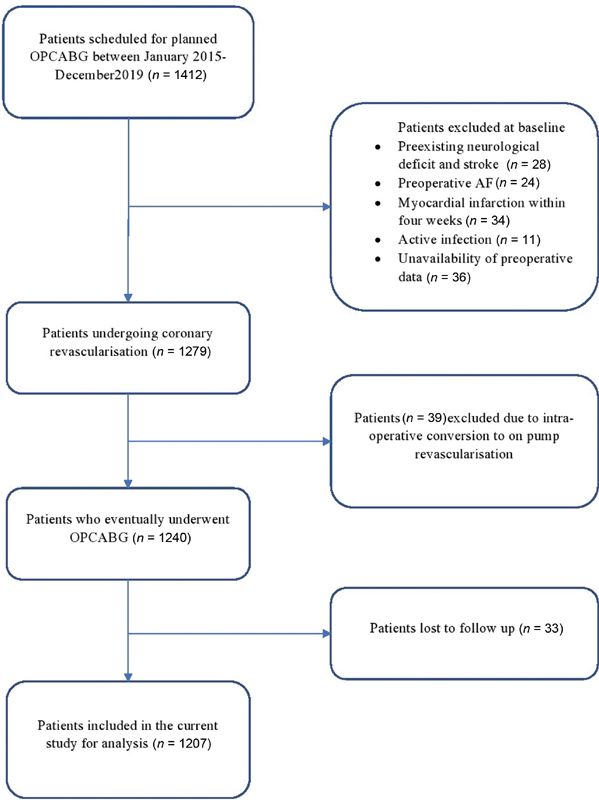
- The flowchart diagram for patient enrolment. AF, atrial fibrillation; OPCABG, off-pump coronary artery bypass grafting.
Preoperative patient characteristics such as age, sex, BMI, WC, European system for cardiac operative risk evaluation (EuroSCORE II), old MI, asymptomatic carotid stenosis, hypertension, diabetes, chronic obstructive pulmonary disease (COPD), history of smoking, preoperative chronic renal failure (CRF), preoperative drugs (statins, β blockers, ACE inhibitors), left ventricle ejection fraction (LVEF), and left main (LM) disease were recorded. The laboratory parameters noted were hemoglobin (Hb), total leucocyte count (TLC), absolute lymphocyte count (ALC), TG, HDL, low-density lipoproteins (LDL), serum creatinine, aspartate transaminase (AST), alanine transaminase (ALT), and serum albumin levels. The intraoperative and postoperative parameters like duration of surgery, number of distal vessel anastomosis, MACCE, postoperative duration of mechanical ventilation (DO-MV), length of intensive care unit stay (LOS-ICU), length of hospital stay (LOS-H), low cardiac output syndrome (LCOS), vasoactive-ionotropic score (VIS), use of IABP, postoperative AF, postoperative acute kidney injury (AKI), and postoperative sepsis were also recorded.
Subsequently, VAI was calculated as per the following formula:
VAI (males) = {WC (cm) × TG × 1.31/ 39.86 + (1.88 × BMI) × 1.03 × HDL}
VAI (females) = {WC(cm) × TG × 1.52/36.58 + (1.89 × BMI) ×1.03 × HDL}
The PNI was computed according to the formula: {10 × albumin(g/dl) +(0.005× total lymphocyte count}.
The CANI values (VAI/PNI) were also characterized from the obtained values of former indices.
The patients' 30-day postoperative data was retrospectively analyzed for the development of MACCE that was defined by any of the following: cardiac arrest, ST segment elevation myocardial ischemia (STEMI), repeat coronary revascularization or stroke.2 STEMI was defined as per the American College of Cardiology Foundation/American Heart Association Task Force guideline.12 LCOS was defined as a decrease in cardiac index to <2 L/min/m2 and a systolic blood pressure of <90 mm Hg, in conjunction with signs of tissue hypoperfusion in the absence of hypovolemia.13 VIS was estimated as per the formula: {VIS= dopamine(µg/kg/min)+ dobutamine(µg/kg/min) + milrinone(µg/kg/min) × 10 + epinephrine(µg/kg/min) × 100 + norepinephrine(µg/kg/min) × 100 + vasopressin(µg/kg/min) × 100 + vasopressin(µg/kg/min) × 1000.14 Postoperative AKI was defined by an increase in serum creatinine value to >3 times of baseline, or increase to absolute value of > 4mg/dL or need for renal replacement therapy (according to stage 3 of Acute Kidney Injury Network (AKIN) classification).15
The anesthetic induction and maintenance were in accordance with a standard institutional protocol. The patients were ventilated using volume control mode with oxygen and air in the ratio of 0.6 to achieve an end tidal carbon dioxide level of 35mmHg alongside a target of 1.0 minimum alveolar concentration (MAC) isoflurane. A Swan-Ganz catheter (Edwards Lifesciences, Irvine, California, United States) was advanced in the pulmonary artery through the 7Fr sheath inserted in the right internal jugular vein and the readings were recorded. The hemodynamic parameters were maintained within 20% of the baseline and a bispectral index (BIS) was maintained between 40 and 60. The intraoperative blood glucose level was maintained between 140 and 180 mg/dL.
After midline sternotomy, left internal mammary artery (LIMA) and saphenous venous graft were harvested. After administering heparin in a dose of 200 IU/Kg of body weight and achieving an activated clotting time of >300 seconds, the octopus evolution tissue stabilizer (Medtronic, Inc., Minneapolis, Minnesota, United States) was used to stabilize the target coronary artery and placing the intracoronary shunts. The distal anastomosis was performed with either 6–0 or 7–0 Prolene sutures, while proximal anastomosis was performed using 5–0 Prolene sutures. Following the completion of coronary anastomosis, heparin was reversed with protamine dose in a ratio of 1 mg/100 IU heparin and all the patients were shifted to cardiac ICU for subsequent management.
Statistical Analysis
The categorical variables were expressed as numbers and percentages and compared between the MACCE and no-MACCE groups employing the chi square test. The continuous variables were expressed as mean ± standard deviation and compared using the unpaired t-test. To study the correlation between continuous variables, Pearson correlation analysis was employed. The nonparametric receiver operating characteristic (ROC) curve analysis was performed to evaluate the accuracy of various variables in predicting MACCE indicated by their respective area under the curve (AUC). The optimum MACCE-predictive cutoff was determined, as the cutoff point with the highest ([sensitivity + specificity]/2) ratio, at which there was the maximal correct categorization of postoperative MACCE as an outcome. The sensitivity, specificity, and predictive values were subsequently reported using the generated cut-offs. The multivariate analysis was performed using binary logistic regression method. The statistical software SPSS version 20 (IBM Corp, Armonk, New York, United States) was employed for the analysis. An α level of 5% with 80% power of study, with a p-value <0.05, considered as significant.
Results
Among the 1,207 patients included, a total of 132 (10.93%) patients developed MACCE in our perioperative OPCABG setting. The demographic variables, perioperative, and laboratory characteristics of the study subjects are presented in Table 1.
|
Variables |
MACCE group (n = 132) |
Non-MACCE group (n = 1,075) |
p-Value |
|---|---|---|---|
|
Preoperative patient characteristics |
|||
|
Age(years) |
66.92 ± 5.02 |
60 ± 8.88 |
<0.001 |
|
Male sex |
106(80.3) |
875(81.4) |
0.761 |
|
BMI (kg/m2) |
21.37 ± 2.37 |
20.94 ± 1.99 |
0.053 |
|
EuroSCORE II |
4.25 ± 0.96 |
4.03 ± 0.98 |
0.015 |
|
Statins |
40(30.3) |
300(27.91) |
0.564 |
|
Beta blocker |
49(37.12) |
324(30.14) |
0.101 |
|
ACE inhibitor |
41(31.06) |
283(26.33) |
0.247 |
|
Prior MI |
30(22.73) |
294(27.35) |
0.258 |
|
LVEF (%) |
40.8 ± 10.01 |
42.7 ± 10.18 |
0.043 |
|
Asymptomatic carotid stenosis |
57(43.18) |
258(24) |
<0.001 |
|
HTN |
80(60.61) |
623(57.95) |
0.56 |
|
DM |
21(15.88) |
110(10.19) |
0.04 |
|
Hyperlipidemia |
75(56.82) |
590(54.88) |
0.673 |
|
Preoperative CRF |
4(3.03) |
53(4.93) |
0.331 |
|
Family history |
20(15.45) |
124(11.53) |
0.194 |
|
COPD |
4(3.03) |
30(2.79) |
0.875 |
|
Smoking |
60(45.45) |
500(46.51) |
0.818 |
|
LM disease |
120(90.91) |
700(65.12) |
<0.001 |
|
Preoperative laboratory parameters |
|||
|
Hb (g/dL) |
12.12 ± 0.68 |
12.16 ± 0.69 |
0.494 |
|
TLC (103/µL) |
7.99 ± 1.87 |
8.12 ± 1.86 |
0.415 |
|
TG (mmol/L) |
2.09 ± 0.16 |
2.06 ± 0.22 |
0.129 |
|
ALC (103/µL) |
1.33 ± 0.34 |
1.31 ± 0.36 |
0.627 |
|
HDL (mmol/L) |
1.10 ± 0.2 |
1.12 ± 0.11 |
0.393 |
|
WC (cm) |
88.51 ± 1.05 |
88.54 ± 2.33 |
0.8 |
|
Urea (mg/dL) |
22.03 ± 3.51 |
21.83 ± 3.32 |
0.456 |
|
Creatinine (mg/dL) |
0.92 ± 0.23 |
0.91 ± 0.23 |
0.796 |
|
Albumin (g/dL) |
3.42 ± 0.17 |
3.44 ± 0.17 |
0.202 |
|
VAI |
3.14 ± 0.78 |
2.69 ± 0.6 |
<0.001 |
|
PNI |
37.8 ± 2.33 |
41.08 ± 2.38 |
<0.001 |
|
CANI |
0.08 ± 0.02 |
0.07 ± 0.02 |
<0.001 |
|
AST (U/dL) |
56.77 ± 14.72 |
60.82 ± 25.63 |
0.075 |
|
ALT (U/dL) |
56.14 ± 14.59 |
59.55 ± 21.32 |
0.086 |
|
Intraoperative and postoperative parameters |
|||
|
Number of grafts |
3.02 ± 0.63 |
2.97 ± 0.45 |
0.252 |
|
Duration of Sx (mins) |
253 ± 30.14 |
249.7 ± 26.34 |
0.277 |
|
Duration of mechanical ventilation(h) |
18.64 ± 4.13 |
15.13 ± 3.19 |
<0.001 |
|
LOS-ICU (d) |
3.44 ± 1.09 |
2.9 ± 0.89 |
<0.001 |
|
LOS-H (d) |
6.83 ± 1.4 |
6.85 ± 1.39 |
0.844 |
|
AF |
30(22.7) |
161(14.9) |
<0.02 |
|
Postoperative AKI |
8(6.06) |
60(5.58) |
0.821 |
|
LCOS |
12(9.08) |
87(6.59) |
0.285 |
|
Mean VIS |
11.45 ± 2.9 |
10.34 ± 3.87 |
0.001 |
|
Postoperative IABP |
5(4.54) |
46(4.18) |
0.846 |
|
Postoperative sepsis |
2(1.51) |
17(1.28) |
0.826 |
|
Mortality |
9(6.82) |
22(2.05) |
0.001 |
Abbreviations: AF, atrial fibrillation; AKI, acute kidney injury, ALC, absolute lymphocyte count; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; CANI, combined adiposity nutritional index; CRF, chronic renal failure; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; DO-MV duration of mechanical ventilation; duration of Sx, duration of surgery; EuroSCORE II, European System for Cardiac Operative Risk Evaluation; HDL, high-density lipoprotein; HTN, hypertension; IABP, intra-aortic balloon pump, LCOS, low cardiac output syndrome; LOS, ICU, length of stay in intensive care unit; LVEF, left ventricle ejection fraction; LM, left main; LOS-H, length of stay in hospital; Hb, hemoglobin; MACCE, major adverse cardiac and cerebral event; SD, standard deviation; TLC; total leucocyte count; MI, myocardial infarction; PNI, prognostic nutritional index; TG, triglyceride; WC, waist circumference; VAI, visceral adiposity index; VIS, vasoactive inotropic score.
Data are presented as either mean ± SD or n (%); p-values < 0.05 are italicized.
On univariate analysis, the following variables were found to be significant predictors of postoperative MACCE: age, EuroSCORE II, DM, LVEF, LM disease, asymptomatic carotid artery disease, VAI, PNI, and CANI (Table 2). On multivariate analysis, age (odds ratio [OR]: 1.121, 95% confidence interval [CI]: 1.087–1.157, EuroSCORE II (OR: 2.557, 95% CI 1: 0.607–4.067), and CANI (OR: 4.12, 95% CI: 0.244–6.96) emerged as independent predictors of MACCE (Table 2).
|
Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
|
Variables |
OR |
95% CI |
p-Value |
OR |
95% CI |
p-Value |
|
Age |
1.111 |
1.082–1.140 |
<0.001 |
1.121 |
1.087–1.157 |
<0.001 |
|
EuroSCORE II |
1.02 |
0.849–1.226 |
<0.015 |
2.557 |
1.607–4.067 |
<0.001 |
|
DM |
1.065 |
0.620–1.830 |
<0.04 |
1.015 |
0.526–1.959 |
0.964 |
|
Asymptomatic carotid stenosis |
2.407 |
1.659–3.490 |
<0.001 |
0.974 |
0.779–1.217 |
0.814 |
|
LVEF |
1.001 |
0.983–1.019 |
<0.043 |
0.999 |
0.977–1.022 |
0.941 |
|
LM disease |
5.357 |
2.921–9.825 |
<0.001 |
1.023 |
1.013–1.033 |
0.891 |
|
PNI |
0.517 |
0.460–0.581 |
<0.001 |
1.851 |
1.517–2.401 |
0.526 |
|
VAI |
2.156 |
1.706–2.725 |
<0.001 |
1.003 |
1.001–2.255 |
0.085 |
|
CANI |
5.06 |
4.06–6.06 |
<0.001 |
4.12 |
0.244–6.96 |
0.041 |
Abbreviations: CANI, combined adiposity nutritional index; CI, confidence interval; DM, diabetes mellitus; EuroSCORE II, European System for Cardiac Operative Risk Evaluation; LM, left main; LVEF, left ventricle ejection fraction; MACCE, major adverse cardiac and cerebral event; OR, odds ratio; PNI, prognostic nutritional index; VAI, visceral adiposity index.
p-Values < 0.05 are italicized.
By ROC analysis, a cutoff of 3.2 for VAI, 38.46 for PNI, and 0.075 CANI predicted MACCE with AUC; sensitivity; specificity of 0.64 (95% CI: 0.59–0.70); 77%; 81.3%, 0.77 (95% CI: 0.75–0.84); 92.6%; 65%, 0.78 (95% CI: 0.66–0.76); 64.5%; 80.2%, respectively (p < 0.001, Fig. 2).
![(A) The comparison of two indices (visceral adiposity index [VAI] and combined adiposity-nutritional index [CANI]) for predicting major adverse cardiac and cerebral event (MACCE) in terms of area under the curve (AUC) under the receiver operating characteristic (ROC) curve depicting the highest AUC of 0.78 for CANI. The respective predictive cutoff values of the parameters have been displayed in the right lower section of the figure. (B) PNI ROC curve for predicting MACCE in terms of AUC depicting the highest AUC of 0.77 for CANI. The predictive cutoff values of the PNI have been displayed in the right lower section of the figure.](/content/149/2021/5/3/img/10-1055-s-0041-1739530-i2190001-2.png)
- (A) The comparison of two indices (visceral adiposity index [VAI] and combined adiposity-nutritional index [CANI]) for predicting major adverse cardiac and cerebral event (MACCE) in terms of area under the curve (AUC) under the receiver operating characteristic (ROC) curve depicting the highest AUC of 0.78 for CANI. The respective predictive cutoff values of the parameters have been displayed in the right lower section of the figure. (B) PNI ROC curve for predicting MACCE in terms of AUC depicting the highest AUC of 0.77 for CANI. The predictive cutoff values of the PNI have been displayed in the right lower section of the figure.
On secondary analysis, it was found that the incidence of postoperative outcomes like DO-MV, LOS-ICU, mean VIS strongly correlated with CANI with Pearson's correlation coefficient (R = 0.86 for correlation with DO-MV, R = 0.62 for correlation with LOS-ICU, R = 0.67 for mean VIS) (Table 3). Interestingly, on categorizing the study subjects as per the MACCE predictive CANI cutoff, it was discovered that that the patients with a CANI≥ 0.075 demonstrated a higher incidence of other postoperative complications such as AF, AKI, and LCOS in contrast to the patients with a CANI < 0.075 (Table 4).
|
r-Value |
p-Value |
|
|---|---|---|
|
DO-MV |
0.86 |
0.003 |
|
LOS-ICU |
0.62 |
0.014 |
|
LOS-H |
0.25 |
0.385 |
|
Mean VIS |
0.67 |
0.035 |
Abbreviations: CANI, combined adiposity nutritional index; DO-MV, duration of mechanical ventilation; LOS-H, length of stay in hospital; LOS-ICU, length of stay in ICU; VIS, vasoactive inotropic score.
R value-Pearson correlation coefficient; p-values < 0.05 are italicized.
|
Total patient population (n = 1,207) |
CANI ≥0.075 (n = 290) |
CANI < 0.075 (n = 917) |
p-Value |
|
|---|---|---|---|---|
|
AF |
191(15.82) |
62 (21.28) |
129 (14.13) |
0.003 |
|
AKI |
68 (5.63) |
27 (9.21) |
41 (4.45) |
0.002 |
|
LCOS |
99 (8.2) |
34 (11.62) |
65 (7.1) |
0.014 |
|
IABP |
51 (4.23) |
17 (5.86) |
34 (3.71) |
0.112 |
|
Postoperative sepsis |
19(1.57) |
8 (2.76) |
11 (1.2) |
0.063 |
Abbreviations: AF, atrial fibrillation; AKI, acute kidney injury; CANI, combined adiposity nutritional index; LCOS, low cardiac output syndrome; IABP, intra-aortic balloon pump.
Data are presented as n (%); p-values < 0.05 are italicized.
Discussion
The index elucidation of an independent MACCE predictive value of CANI in our OPCABG setting is remarkable with regard to the pertinent need of objectifying the metabolic–nutritional profile of CAD patients presenting for surgical revascularization. While a wide range of postoperative MACCE incidence has been described in the existing literature, the follow-up period varies substantially.1, 2, 3 Nevertheless, a 30-day MACCE incidence of 10.93% in our study relates closely to the Kaplan–Meier time-stamped cumulative event curves for MACCE prediction in the 5-year follow-up period of the randomized clinical SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) trial.1
The individual components of the novel proposed CANI, that is, VAI and PNI, have been studied across diverse clinical settings and heterogeneous patient population.5, 16, 17, 18, 19 However, the alterations of the above-mentioned indices in CAD patients have captivated recent research attention.5, 20, 21, 22 At the same time, literature is accumulating on the possible role of VAI and PNI in predicting cardiac surgical outcomes, particularly in settings of coronary revascularization.10, 11, 23 Engin et al10 outline an escalated risk of post-CABG AF in patients with a VAI higher than 3.59 (AUC: 0.73, 84.6% sensitivity and 53.1% specificity) and a PNI less than 43.7 (AUC: 0.68, 76.4% sensitivity and 48.3% specificity), whereas the MACCE predictive cutoffs of VAI and PNI were 3.2 and 38.46, respectively, in our study.
Talking specifically of MACCE (primary outcome under evaluation), independent researchers describe an association between increased VAI values and adverse cardiovascular and cerebrovascular events in nonoperative settings.5, 24 Moreover, the retrospective analysis of 374 cardiac surgical patients by Lee et al highlights a considerably higher postoperative morbidity–mortality in background of a PNI < 46.13.11 Alongside the MACCE-predictive potential of CANI, the strong positive correlation of the former with DO-MV, LOS-ICU, and mean postoperative VIS elucidated in the present study is equally noteworthy. In congruence with the Dolapoglu et al23 and Yost et al25 description of a PNI predictive value of AKI and a PNI-based prognostication in left ventricular support device, respectively, we discovered a higher incidence of postoperative complications like AF, AKI, and LCOS in patients with a CANI ≥ 0.075.
Interestingly, the independent MACCE predictive value of CANI (ahead of the VAI and PNI) as a primary finding of our study is in agreement with our hypothesis that a patients' snapshot nutritional status can be rendered more meaningful with the incorporation of the baseline cardiometabolic risk. It is worthwhile to mention that the syndrome X patients in the index analysis (overall incidence: 28.9% [22.7% in non-MACCE group and 79.5% in MACCE group]) demonstrated a 43% increase in the estimated mean CANI values in contrast to the nonsyndrome X patients.
While the pivotal role of visceral adiposity in determining outcomes deserves attention amidst ardent debates on “obesity-paradox,”26 the simultaneous account of hypoalbuminemia and lymphocytopenia (included in PNI) presents the potential of deciphering the risk predisposition owing to a possible perioperative metabolic–nutritional-inflammatory cross-talk.27, 28 The recommendation on preoperative albumin level testing and correction of nutritional deficits as per the recent guidelines for an enhanced recovery after cardiac surgery (ERACS)27 exemplifies the aforementioned and suggests a dynamic role of preoperative composite indices, such as CANI.
To the best of our knowledge, the index research endeavor is first of a kind in investigating the MACCE predictive potential of nutritional and metabolic indices in an isolated subset of OPCABG patients. While a large homogeneous patient cohort classifies as the biggest study strength, the prognostic potential of metabolic–nutritional patient profile is often overlooked11 and can be particularly pertinent in setting of a developing world. Owing to an isolated OPCAB patient subset, we could effectively eliminate the potential confounding effects of hemodilution, cellular-lysis, inflammatory, and coagulation pathway activation inherent to on-pump coronary revascularization.29 At the same time, employing objective definitions of all the postoperative outcomes under evaluation adds an incremental value.
The study, however, is inherently limited by its retrospective design that is susceptible to residual confounding.30 The exclusion of patients on mechanical ventilation, preoperative IABP, AF, etc. who have high baseline predisposition to development of MACCE might have caused leftward skewed data results. Moreover, a postoperative follow-up limited to a duration of 30 days could have also compounded the matter. While a single-center study bestows cohort homogeneity, the research findings can only be generalized awaiting external validation and future large-scale prospective studies. Lastly, lack of formal objective data on frailty in the retrospective analysis presents an additional limitation.
Conclusion
CANI independently predicted MACCE in our patients undergoing OPCABG. The novel parameter can emerge as a simple, parsimonious, objective measure of metabolic–nutritional risk profile. In addition, it may potentially serve as an important performance metric of the cardiac prehabilitation programs, which are in particular vogue in the present era of ERACS.31
Conflict of Interest
The authors declare no competing interests.
Funding Support was provided solely from institutional and/or departmental sources.
References
- Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629-638. (9867):
- [Google Scholar]
- Red blood cell distribution width predicts long term cardiovascular event after on-pump beating coronary artery bypass grafting. J Cardiothorac Surg. 2016;11:48.
- [Google Scholar]
- Can red blood cell distribution width predict long-term cardiovascular event after off-pump coronary artery bypass? A retrospective study. J Card Surg. 2019;34(10):988-993.
- [Google Scholar]
- Red blood cell distribution width and outcomes following coronary artery bypass grafting: looking back and forth. Indian J Thorac Cardiovasc Surg. 2020;36(2):168-169.
- [Google Scholar]
- Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920-922.
- [Google Scholar]
- Visceral adiposity index: an indicator of adipose tissue dysfunction. Int J Endocrinol. 2014;2014:730827.
- [Google Scholar]
- [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients] Nippon Geka Gakkai Zasshi. 1984;85(9):1001-1005.
- [Google Scholar]
- The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140(9):1537-1549.
- [Google Scholar]
- Immunonutritive scoring in patients with hepatocellular carcinoma undergoing transarterial chemoembolization: prognostic nutritional index or controlling nutritional status score? Front Oncol. 2021;11:696183.
- [Google Scholar]
- Visceral adiposity index and prognostic nutritional index in predicting atrial fibrillation after on-pump coronary artery bypass operations: a prospective study. Rev Bras Cir Cardiovasc. 2020;••• (ahead of print)
- [CrossRef] [Google Scholar]
- Does the prognostic nutritional index have a predictive role in the outcomes of adult cardiac surgery? J Thorac Cardiovasc Surg. 2020;160(1):145-153.e3.
- [Google Scholar]
- 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362-e425.
- [Google Scholar]
- Low-cardiac-output syndrome after cardiac surgery. J Cardiothorac Vasc Anesth. 2017;31(1):291-308.
- [Google Scholar]
- Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11(2):234-238.
- [Google Scholar]
- Effect of goal-directed therapy on post-operative neutrophil gelatinase-associated lipocalin profile in patients undergoing on-pump coronary artery surgery. Indian J Thorac Cardiovasc Surg. 2019;35(3):445-452.
- [Google Scholar]
- Visceral adiposity index is associated with insulin sensitivity and adipocytokine levels in newly diagnosed acromegalic patients. J Clin Endocrinol Metab. 2012;97(8):2907-2915.
- [Google Scholar]
- Visceral adiposity index (VAI) is predictive of an altered adipokine profile in patients with type 2 diabetes. PLoS One. 2014;9(3):e91969.
- [Google Scholar]
- Onodera's prognostic nutritional index as a risk factor for mortality in peritoneal dialysis patients. J Korean Med Sci. 2012;27(11):1354-1358.
- [Google Scholar]
- Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur J Surg Oncol. 2015;41(11):1508-1514.
- [Google Scholar]
- Visceral adiposity in relation to body adiposity and nutritional status in elderly patients with stable coronary artery disease. Nutrients. 2021;13(7):2351.
- [Google Scholar]
- A novel and useful predictive indicator of prognosis in ST-segment elevation myocardial infarction, the prognostic nutritional index. Nutr Metab Cardiovasc Dis. 2017;27(5):438-446.
- [Google Scholar]
- Prognostic nutritional index predicts clinical outcome in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Sci Rep. 2017;7(1):3285.
- [Google Scholar]
- The predictive value of the prognostic nutritional index for postoperative acute kidney injury in patients undergoing on-pump coronary bypass surgery. J Cardiothorac Surg. 2019;14(1):74.
- [Google Scholar]
- Visceral adiposity and risk of coronary heart disease in relatively lean Chinese adults. Int J Cardiol. 2013;168(3):2141-2145.
- [Google Scholar]
- Preoperative nutritional assessment with the prognostic nutrition index in patients undergoing left ventricular assist device implantation. ASAIO J. 2018;64(1):52-55.
- [Google Scholar]
- Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640-1649. (9497):
- [Google Scholar]
- Guidelines for perioperative care in cardiac surgery: enhanced recovery after surgery society recommendations. JAMA Surg. 2019;154(8):755-766.
- [Google Scholar]
- Systemic immune-inflammation index predicts poor outcome after elective off-pump CABG: a retrospective, single-center study. J Cardiothorac Vasc Anesth. 2021;35(8):2397-2404.
- [Google Scholar]
- Endothelial glycocalyx and cardiac surgery: newer insights. J Cardiothorac Vasc Anesth. 2020;34(1):310-311.
- [Google Scholar]
- Enhanced recovery after cardiac surgery: is it just about putting the bundles together? Ann Card Anaesth. 2021;24(2):276-278.
- [Google Scholar]







