Translate this page into:
Extracorporeal Membrane Oxygenation for Lung Transplantation
Poonam Malhotra Kapoor, MD, DNB, MNAMS, FISCU (Hony), FIACTA (Hony), FTEE (Hony) Department of Cardiac Anaesthesia and Critical Care, All India Institute of Medical Sciences New Delhi 110029 India drpoonamaiims@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Introduction
Extracorporeal membrane oxygenation (ECMO) has a clearly defined role in preoperative and postoperative transplant management of both heart and lung transplant. It is used on the recipient as a bridge to transplant, until the organs are made available. It provides a good support during surgery as a partial cardiopulmonary bypass. Post-transplant, it again provides support in case of an acute rejection or when graft dysfunction occurs, postoperatively. When ECMO as an indication is continued to provide support. Thus, ECMO has a positive impact on lung transplant outcomes.1 In a randomized control study, known as the CAESAR TRIAL in 2009, ECMO was confirmed as a definitive modern of treatment for ARDS.2 It gained the popularity for treating adult ARDS. Veith et al in 19773 reported that can be used as a support or as a bridge to lung transplant, with both short-term and long-term results. Thus, today in 2022, ECMO has emerged as a preferred modality of intraoperative support over conventional CPB.4 It was the reports of successful therapy of post graft dysfunction (PGD) with ECMO that prompted the use of ECMO intraoperatively. Currently, ECMO for lung transplant patients is the only rescue therapy to salvage a graft affected by severe PGD.
Preoperative use of ECMO as a Bridge to Lung Transplant
Earlier, ECMO and mechanical ventilation were considered major contraindications to lung transplant.5 For ECMO to serve as a bridge to lung transplant, resources of prolonged ICU and hospital steps with frequent transfusions and their complications are required, and one must weigh all consequences of this modality as the rehabilitation required is also prolonged post hospital discharge.
Indications and Contraindications of ECMO for lung transplant
It is indicated in irreversible end-stage lung diseases presenting with:
-
Rapid deterioration of respiratory status as reflected by refractory hypercapnia and/or hypoxic respiratory failure (usually pCO2 > 80 mm Hg and P/F < 80 mm Hg).
-
Severe pulmonary hypertension and hemodynamic collapse due to severe dysfunction of the right ventricle.
-
Contraindications for the use of ECLS are septic shock, multi organ dysfunction, severe arterial occlusive disease, and heparin-induced thrombocytopenia type II.
Choice of Mode of Extracorporeal Life Support in Lung transplant
The mode of ECLS mainly depends on the indication for ECMO support. The usual indications for ECMO support are type II respiratory failure with respiratory acidosis, type I respiratory failure, and severe pulmonary hypertension with right ventricular failure. In all three types, the mode of ECLS is different (Fig. 1).
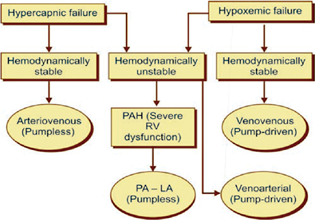
- Choice of ECLS modes for pre-transplant lung support.
Refractory Hypercapnia Respiratory Failure and Acidosis
For type II respiratory failure with severe hypercapnia and acidosis, interventional lung assist device (iLA, Nova lung, Germany) remains a good option. This allows prompt and effective CO2 removal and correction of respiratory acidosis. To use a pump-less device, the patient must be hemodynamically stable and should have a mean arterial blood pressure of > 80 mm Hg to sustain good flows through the device. It is not recommended for patients with severe hypoxia (paO2/FiO2 < 80 mm Hg).6
Hypoxemic Respiratory Failure
For a patient with predominant hypoxic failure, one has to go for the pump-driven ECLS support with PMP oxygenator. If the patient is hemodynamically stable, then VV mode is the preferred choice. However, if the patient is hemodynamically compromised requiring high inotropic support, then VA ECLS is the recommended option because it provides both cardiac and pulmonary support.
Pulmonary Hypertension and Right Ventricular Failure
A patient with severe primary pulmonary hypertension with right ventricular failure a low resistance gas exchange device (Nova lung) between the main trunk of the pulmonary artery and the left atrium (PA-LA) in a pump-less mode is most effective. The elevated pressure in the pulmonary arteries serves as the driving force for the device and obviates the need for a pump. Extubating, physiotherapy, and ambulation are achievable while a patient is on pump-less PA-LA ECLS awaiting a compatible donor lung. Another innovative ECLS approach to bridge patients with severe PAH to lung transplantation is VV ECLS with added atrial septostomy.
The use of ECMO to support the recipient during the transplant procedure has several advantages. These are enumerated in Table 1.
|
|
Management of ECMO During Lung Transplant
During VV ECMO support, a protective ventilator strategy (FiO2 < 30%, pressure control < 22, PEEP 8, rate < 10) is used including low-oxygen, low-pressure ventilation as shown in Fig. 2.
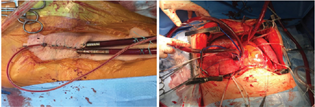
- VV ECMO perfusion during lung transplant.
Extracorporeal Membrane Oxygenation in Postlung Transplant Patients
As PGD may lead to early mortality, its incidence should be noted early, and it is here that VV ECMO, if it is initiated early, there is marked improvement in hemodynamic instability, organ function, and cardiac recovery.
As ECMO is initiated, there is a marked decrease in the pulmonary vascular resistance. Also, with VV ECMO, the capillary leak usually settles down within 24 hours, which is not the case with venoarterial ECMO, which resolves over 48 to 72 hours. Pulmonary compliance needs to be built up on ECMO; so, repeated bronchoscopies are done to remove the secretions and edema. Many authors have reported the superiority of VV ECMO over VA ECMO for the correction of PGD post lung transplant.8 More than the type of ECMO (VA or VV ECMO), it is the timing of the initiation of ECMO, after transplantation, that remains the most important predictor of the ECMO for PGD post lung transplant outcomes. Lung protective ventilation and peripheral cannulation of VV ECMO initiation, at the time of lung transplantation, have been reported by most authors,9 who prefer to withhold anticoagulation at ECMO initiation, until adequate hemostasis of lung transplantation is achieved. In addition, recipient coagulopathy should be urgently corrected before initiating ECMO for lung transplant. (Table 2).
|
Problem |
Assessment |
Management |
|---|---|---|
|
When there is impaired gas exchange |
• Then assessment for: • PGD, • large • hemothorax because of kinked drain, • pulmonary embolism, • auto PEEP associated with single-lung transplant for COPD, • obstructed pulmonary veins, • pneumonia, • bronchial anastomotic leak. |
• Check ventilator settings. • Perform chest radiograph, chest ultrasound, • bronchoscopy, • TOE CT angiogram if • pulmonary embolism is suspected. • VV ECMO, if progressive. |
The access for VV cannulation is dependent on the size of the recipient, the available large vein access sites, the urgency of establishing ECMO, and timing related to the transplant procedure. Our preference is to perform peripheral cannulation. When ECMO is initiated either at the time of transplant or soon thereafter, anticoagulation is not initiated until reasonable hemostasis is achieved. Frequently, recipient coagulopathy must be corrected. When chest tube drainage is acceptable (<50 mL/h), heparin is initiated targeting an ACT of 150 to 200 seconds. Weaning from ECMO is done when the patient's lung compliance has returned to acceptable levels, the pulmonary edema has resolved, the chest X-ray has demonstrated predominant resolution of the infiltrates, and acceptable gas exchange is achievable with moderate ventilator support. Weaning from VV ECMO involves discontinuing membrane gas flow and increasing ventilator parameters as needed.
ECMO in lung transplant is required in up to 30% to 40% of procedures. Tt allows for hemodynamic stability and larger degree of flexibility in surgical manipulation while performing the surgical anastomoses and also aids ventilation and prevents troubleshooting.
Conclusion
VV ECMO can be used as a bridge to lung transplantation with comparable short-term and mid-term outcomes. Newer cannulation strategies with dual-lumen single cannula allow for ambulatory VV ECMO with patients being able to participate in physical therapy and rehabilitation before transplantation. Patients with severe forms of PGD after lung transplantation and who are on maximal ventilator support (peak inspiratory pressures 30 cm H2O, FiO2 > 0.6) should be considered for VV ECMO.
This special issue with ECMO as main there, a special edition, dedicated to the use of ECLS modality in cardiac and respiratory failure. A special editorial by the ELSO stalwart members and of SWAACELSO makes it experts writing on the new stethescope -ECMO which post covid-19 pandemic is used in global ICU's, especially in cardiac critical care.
A special acknowledgement to Professor Randeep Guleria, our AIIMS Director and the AIIMS team for successfully continuing “ECMO” in many AIIMS ICU's and initiating the lung transplant programme at AIIMS.
Conflict of Interest
None declared.
References
- Extracorporeal membrane oxygenation and lung transplantation. Indian J Thorac Cardiovasc Surg. 2021;37(02):327-337.
- [CrossRef] [Google Scholar]
- CESAR: conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res. 2006;6:163.
- [CrossRef] [Google Scholar]
- Bilateral lung transplantation on intraoperative extracorporeal membrane oxygenator: An observational study. J Thorac Cardiovasc Surg. 2020;160(1):320-327.e1.
- [Google Scholar]
- Extracorporeal membrane oxygenation as a bridge to lung transplant: considerations for critical care nursing practice. Crit Care Nurse. 2020;40(3):49-57.
- [Google Scholar]
- Bridge to lung transplantation with the novel pumpless interventional lung assist device NovaLung. J Thorac Cardiovasc Surg. 2006;131(3):719-723.
- [Google Scholar]
- Replacing cardiopulmonary bypass with extracorporeal membrane oxygenation in lung transplantation operations. Eur J Cardiothorac Surg. 2007;31(3):462-467. , discussion 467
- [Google Scholar]
- Improved results treating lung allograft failure with venovenous extracorporeal membrane oxygenation. Ann Thorac Surg. 2005;80(5):1872-1879. , discussion 1879–1880
- [Google Scholar]
- Recipient selection, timing of referral, and listing for lung transplantation. Indian J Thorac Cardiovasc Surg. 2022;38(02):237-247.
- [Google Scholar]






