Translate this page into:
A Randomized Controlled Trial to Evaluate the Use of Probiotics in Prevention of Ventilator-Associated Pneumonia in Critically Ill ICU Patients
Ravi Anand, DNB, FACEE Department of Trauma and Emergency, Indira Gandhi Institute of Medical Sciences Patna, Bihar, 800014 India drravianand75@yahoo.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context Ventilator-associated pneumonia (VAP) is one of the most common causes of morbidity and mortality in mechanically ventilated patients. Curing and preventing effects of probiotics in promoting the growth of Bifidobacterium in the digestive system and the central role of bacteria colonization in the pathogenesis of VAP are evident.
Aims The purpose of this study was to evaluate the effects of administration of commercially available probiotics, that is, orodispersible probiotic sachets on VAP prevention and clinical outcomes in critically ill patients.
Settings and Design Randomized control trials.
Methods and Materials In this study, 120 mechanically ventilated patients were randomly divided into two groups (n = 60 per group). Group 1 was given orodispersible probiotic sachets by gavage, twice a day in addition to routine care, while group 2 received only routine care. Demographic and clinical data were analyzed and clinical outcomes to the primary component (prevalence of VAP) and secondary component (other clinical factors) were interpreted.
Statistical Analysis Used In this study, data were analyzed via SAS statistical software version 9.4, using Student's t-test, chi-squared test, repeated measure analysis of variance, and Wilcoxon test.
Results There was a significant reduction in VAP diagnosed patients, as well as Clostridium difficile-associated diarrhea and some complications of mechanical ventilation, in group 1 in comparison to group 2. The improvement in VAP was significantly greater for group 1 as compared with group 2. However, the mortality rate was similar between two groups.
Conclusions This study demonstrated that a daily diet with orodispersible probiotic sachets can be used as add-on therapy with other medications in the prevention of VAP. As a result, the use of orodispersible probiotic sachets in the treatment plan of patients undergoing long-term intubation is recommended.
Keywords
ventilator-associated pneumonia
probiotics
diarrhea
Key Messages
Diet with orodispersible probiotic sachets can be used as add-on therapy with other medications in the prevention of VAP.
Introduction
Ventilator-associated pneumonia (VAP) is one of the most common causes of morbidity and mortality in mechanically ventilated patients.1 It usually develops after 48 hours or longer after mechanical ventilation (MV). The pathogenesis of VAP is complex and usually requires bacterial colonization of the upper digestive tract and aspiration of contaminated secretions into the lower airway.2
The various trials and studies are done to determine the best pharmacological preventive strategies inhibiting the colonization of the micro-organisms such as the use of antibiotics for selective digestive decontamination (SDD) or selective oral decontamination (SOD) or the utilization of probiotics.
The use of probiotics has been shown to have a promising effect in many randomized controlled trials1, 2 and the use of antibiotics for SDD or SOD has been associated with an increase in antibiotic resistance and cost.3 Probiotics are live nonpathogenic microbes that reduce bacterial translocation by activating mucosal immunity and regulating the release of proinflammatory cytokines. Various mechanism utilized to inhibit the growth of microorganism by probiotics such as organic acid, hydroperoxide and bacteriocins, competition for nutrients, inhibition of pathogen attachment, and inhibition of the action of microbial toxins.4 Probiotics also stimulate the proliferation of the traditional epithelium that helps maintain the mucosal defense barrier.5
The purpose of this study was to determine the effect of probiotic use in critically ill adult patients on MV in the incidence of VAP, length of hospital stay, length of intensive care unit (ICU) stay, duration of MV, the incidence of diarrhea, and the incidence of oropharyngeal colonization and in-hospital mortality.
Materials and Methods
After approval from institutional ethical committee (IEC) of Indira Gandhi Institute of Medical Sciences, Patna, with letter no. 81/IEC/IGIMS/2021 dated:23/03/2021, 120 patients were selected for the double blinded, randomized study during April 2021 to March 2022 in trauma and emergency ICU. Written informed consent was obtained from first degree relative to all enrolled patients.
The inclusion criteria were included in all critically ill patients of at least 18 years of age who were mechanically ventilated with an endotracheal tube for more than 48 hours.
The exclusion criteria during study were included as severe multiple organ failure, with an Acute Physiology and Chronic Health Evaluation (APACHE) II score of more than or equal to 25; MV for more than 72 hours prior to enrolment; Failure of enteral feeding; administration of immune-depressants 1 week before enrolment or diagnosis of immunosuppressive diseases, such as malignant tumor, acquired immune deficiency syndrome and human immunodeficiency virus carriers, pregnancy or lactation.
Study Design
Each patient who met the inclusion criteria was enrolled, and all the benefits and expected complications were explained to the first-degree relative by the primary investigator. Then each patient was randomly assigned by computer to one of two groups; group 1 (probiotic group) and group2 (control group). Sample size of group1 and group 2 consisted of 60 patients each.
Demographic information and baseline clinical data (gender, age, medical history, APACHE II score) were collected on the first day of admission (Table 1). Patients participated in the study until extubation, tracheotomy, discharge, or death occurred. All the enrolled patients received all VAP-preventive measures throughout the study. The protocol remained unchanged during the study. All who involved were blinded from the study.
|
Variables |
Group (n = 60) |
Group 2 (n = 60) |
p-Value |
|---|---|---|---|
|
Gender: Male |
24 (40) |
38 (63.3) |
0.120* |
|
Female |
36 (60) |
22 (36.6) |
|
|
Age (mean ± SD) |
55.465 ± 17.28 |
56.623 ± 14.371 |
|
|
APACHE II score (mean ± SD) |
22.7 ± 7.5 |
23.7 ± 8 |
0.45** |
|
Head trauma |
16 (26.6) |
14 (23.3) |
0.792* |
|
Multiorgan trauma |
9 (15) |
7 (11.7) |
0.771* |
|
Diabetes disease |
19 (31.6) |
21 (35) |
0.90* |
|
Respiratory failure |
16 (26.6) |
18 (30) |
0.27* |
|
Reasons for ICU Admission |
|||
|
Medical |
32 (53.3) |
30 (50) |
0.792* |
|
Scheduled surgery |
18 (30) |
16 (26.6) |
0.890* |
|
Unscheduled surgery |
03 (5) |
04 (6.7) |
0.89 * |
|
Trauma |
07 (11.7) |
10 (1.7) |
0.89 * |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit; SD, standard deviation.
p-Value for comparison between the two groups (p < 0.05). 2 test
This clinical trial was designated as a prospective, open-label, randomized, controlled study. The probiotic group was given commercially available probiotics, that is, orodispersible probiotic sachet that consists Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium longum, and Saccharomyces boulardii twice daily and the standard preventive strategies of VAP were done. VAP preventive measures included in this study were daily screening for weaning potential and weaning from MV as early as possible, hand hygiene, head up position (30–40 degrees), perilaryngeal suctioning to prevent micro aspiration, daily oral care with chlorhexidine (0.12–2%) solution. The control group was only given standard preventive strategies.
For the diagnosis of VAP, all patients were evaluated daily for the presence of VAP by the principal investigator of the study. A clinical diagnosis of VAP was made on the basis of the presence of a new, persistent or progressive infiltrate on chest radiographs that persist for at least 48 hours (as interpreted by radiologists blinded to the patients' treatment assignments) combined with at least two of the following criteria: temperature of >38.0 C or <35.5 °C; a blood leukocytosis count of >12 × 103/mm3 or <3 × 103/mm3; purulent tracheal aspirates.
The physician excluded other pulmonary diseases such as acute respiratory distress syndrome, lung edema, pulmonary tuberculosis, pulmonary embolism, cryptogenic organizing pneumonia, and acute interstitial pneumonia before making a clinical diagnosis of VAP. All clinical diagnoses of VAP were evaluated and endotracheal aspirate samples for semiquantitative cultures of peptide-conjugated phosphorodiamidate morpholino oligomer were obtained from all patients with clinically diagnosed VAP. These cultures were scored using the four-quadrant method, with a score of 0 indicating no growth; 1+ = rare growth; 2+ = light growth; 3+ = moderate growth; 4+ = heavy growth. A score of 3+ or 4+ defined the presence of microbiologically confirmed VAP in the semiquantitative cultures of endotracheal aspirate.
Early-onset VAP was defined as VAP diagnosed within the first 4 days of MV, and late-onset VAP was diagnosed when VAP presented thereafter. All microbiology laboratory people who involved in this study were blinded to the study.
Data Collection
The age, sex, medical specialty, diagnosis at admission, reason for MV, prior antibiotic use, length of hospital stays before admission to ICU, and APACHE II scores (range: 0–71) of each patient were recorded at baseline.
The primary outcome of this study was to evaluate the incidence of VAP.
Secondary outcomes included in the study were the average of days spent in the ICU, length of stay in hospital, and rates of Clostridium difficile-associated diarrhea, cases of constipation, mean gastric residual volume in first 2 days, analysis of respiratory aspiration, and duration of MV in days.
Sample Size and Statistical Analysis
We calculated that a sample size of 120 patients (60 patients per group) were required for the study to have 90% power to show a 50% relative reduction in VAP at a one-sided Alfa level of 0.05. Statistical analysis was performed using SAS statistical software version 9.4 (SAS Institute Cary, North Carolina, United States). Qualitative variables were compared between the two groups using Student's t-test, chi-squared test, repeated measure analysis of variance, and Wilcoxon test. All p-values were one sided and p < 0.05 were considered statistically significant.
Results
Of the 120 patients (100%) (group 1, n = 60; group 2, n = 60) that were randomly selected, 18 patients (15%) were excluded during the study. Out of these 18 patients (100%), 8 (44.44%) and 10 (55.55%) patients were from group 1 and group 2, respectively. In group1, out of 8 (100%) excluded patients, 4 (50%) patients died in the first 48 hours of ventilatory support and 4 (50%) patients were extubated because of unlikely to need MV for at least 48 hours. Out of the 10 (100%) excluded patients in group 2, three (30%) patients died in the first 48 hours of ventilatory support, four (40%) patients withdrawn their consent to continue the study, and three (30%) patients extubated because of unlikely to need MV for at least 48 hours. SAS statistical software version 9.4 (SAS Institute Cary, North Carolina, United States) was used for statistical analysis.
To compare differences between the groups, Student's t-test (normal distribution) and Mann–Whitney U test (non-Gaussian distribution) were used for continuous variables, and chi-squared (_2) test was used for categorical variables. The mean age (p = 0.25), mean sex (p =0.120), mean APACHE II score (p = 0.65), risk factors of VAP (p = 0.56), reasons for admission to the ICU (p = 0.120), and oral hygiene status (p = 0.3) were all noted. There were no significant differences between the two groups in the primary admission.
The primary outcomes of this study was to evaluate the incidence of VAP, as analyzed by generalized Wilcoxon test. Of the 120 patients, only 102 completed the study and out of 102 patient, 44 patients were diagnosed with VAP (group 1: 14 patients [26.9%]; vs. group 2: 30 patients [60%]; p = 0.03) (Fig. 1). In this study, the addition of orodispersible probiotic sachets in the daily diet led to a significant reduction in cases of VAP.
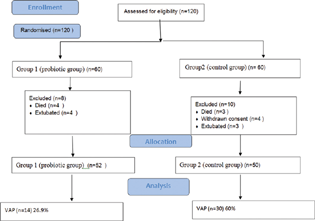
- Patients analyzed for the primary end point. VAP, ventilator-associated pneumonia.
Secondary outcomes included the average of days spent in the ICU (group 1: 14.2 ± 4.7 vs. group 2: 17.6 ± 6.5; p = 0.028), length of stay in hospital (group 1: 24.1 ± 5.6 vs. group 2: 27.4 ± 6.6; p = 0.041], and rates of Clostridium difficile-associated diarrhea (group 1: 2 [3.33%] vs. group 2: 12 [20%]; p = 0.04) were different between the two groups. In first 2 days, the mean gastric residual volume was almost same between the two groups, but after 2 days, there was a strong trend to decrease in group 1 (p = 0.001)). Analysis of cases of respiratory aspiration (3.3 vs. 33.3% for group 1 vs. group 2; p = 0.005) and mean time to occurrence of VAP (6.1 ± _2.6 vs. 10.5 ± _1.02 for group 1 vs. group 2; p = 0.008) revealed that they are higher in the group 2. Duration of MV in days (10.26 ± 6.05 vs. 12.06 ± 4.81 for group 1 vs. group 2; p = 0.64) was not different between the groups. No side effects attributable to the use of commercially available probiotics (orodispersible probiotic sachets) were observed. Specifically, no cases of diarrhea related to ICU were seen. There were three cases of constipation seen in group 1, while both cases were significantly increased in group 2, p = 0.001 (Table 2).
|
Variables |
Group 1 |
Group 2 |
P-value |
|---|---|---|---|
|
Stay in ICU (d) |
14.2 ± 4.7 |
17.6 ± 6.5 |
0.028*** |
|
Length of stay in hospital(d) |
24.1 ± 5.6 |
27.4 ± 6.6 |
0.041*** |
|
Duration MV (d) |
10.26 ± 6.05 |
12.06 ± 4.81 |
0.64** |
|
Time onset VAP (d) |
10.5 ± 1.21 |
6.1 ± 2.64 |
0.008*** |
|
Gastric residual volume (mean ± SD) |
33.108 ± 09.15 |
81.16 ± 58.24 |
0.001** |
|
ICU-associated diarrhea |
0 |
4 (8) |
0.001* |
|
Clostridium difficile-associated diarrhea |
1 (1.92) |
6 (12) |
4.0* |
|
Respiratory aspiration of fifth and sixth days |
1 (1.92) |
10 (20) |
0.005**** |
|
Constipation |
3 (5.7) |
25 (50) |
0.001* |
|
Normal defecation |
47 (90.38) |
5 (10) |
0.001* |
|
Clinical status of patients' recovery |
|||
|
Complete recovery |
42 (80.76) |
32 (64) |
0.003* |
|
Relative recovery |
7 (13.46) |
8 (16) |
0.38* |
|
Failure recovery |
3 (5.7) |
10 (20) |
0.04* |
Abbreviations: ICU, intensive care unit; MV, mechanical ventilation; SD, standard deviation; VAP, ventilator-associated pneumonia.
p-Value for comparison between the two groups (p < 0.05). *2 test, **independent t test, ***Mann–Whitney test, ****Cochrane analysis test.
In relation to the clinical situation of patients, the results showed that complete recovery (83.3 vs. 46.6%; p = 0.028] was significantly higher in group 1. Lack of recovery was significantly lower in group 1 (3.3 vs. 20%; p = 0.04). Although the mortality rate was lower in group 1 than group 2 (2.2 vs. 20%; p = 0.12), there was no significant difference between the two groups.
Discussion
This study demonstrated that the use of orodispersible probiotic sachets has favorable effects on VAP, Clostridium difficile-associated diarrhea, and other clinical outcomes (e.g., MV duration and length of stays in ICU). However, the changes in mortality are negligible. These findings were slightly differed on the findings of other scientists. Johnstone et al in their meta-analysis found that probiotics do not show any beneficial effect on VAP.1
Probiotics may be ineffective before persisting in the lower digestive tract.2 Probiotics cannot be general effects and their viability in gut environment is different. Probiotics are nothing but a form of dietary fiber that cannot be digested and good bacteria present in any individual's gut.3, 4, 5 Side by side, administration of some probiotic strains in severe acute pancreatitis did not reduce the risk of infectious complications and was associated with an increased risk of mortality.6, 7
Torres et al observed that the probiotic cells microencapsulated with alginate fenugreek gum-locust matrix have enhanced viability than nonencapsulated cells during storage time of 3 months at 4 °C. Probiotics tolerated digestive condition was efficient compared to nonencapsulated bacteria. These results showed that orodispersible probiotic sachets act as a probiotic.4 Subsequently, the balance of gut flora enhances the innate immune system, which is associated with a reduction in the risk of VAP. Thus, the above results suggest and confirm that orodispersible probiotic sachets diet may be promising for the management of critically ill VAP patients.
Weng et al conducted a meta-analysis involving 844 patients (423 in the prebiotic group and 421 in the control group) in relation to the use of probiotics to prevent VAP.8 The results of their meta-analysis study concluded that VAP was reduced in the probiotic group, as compared with the control group. However, their difference was not significant, even over a total of five studies.9, 10, 11, 12, 13 Most of the results of various meta-analysis (e.g., ICU stay, hospital stay, duration of MV, and diarrhea) were consistent with our results.
Our study is unique in that it demonstrates that use of orodispersible probiotic sachets can significantly reduce residual volume and respiratory aspiration, and eventually lead to a reduction in VAP. Our study also differs from previous studies in that we conducted a double-blinded study and promoted the growth of good bacteria specific to each person's gut by the use of commercially available probiotics, that is, orodispersible probiotic sachets.
This study had multiple limitations. First, the result of secondary end points, sample size was not of a large cohort. Second, the data were obtained from a single territory with innate prejudices related to local habits and population. Third, we should include those patients who were expected to have been under ventilation for at least 48 hours with a high risk of VAP. These issues indicate that the results of this study cannot be forced to compliance all ICU patients. Truth is that the data interpretation is mainly for patients with a high risk of VAP. Collectively, these data suggest that use of orodispersible probiotic sachets may be safe, nutritional, inexpensive, and a nonantibiotic procedure to prevent VAP in ICU patients. As a result, use of orodispersible probiotic sachets in the diet of patients undergoing long-term intubation is recommended.
Conclusion
In this study, we demonstrated the effect of orodispersible probiotic sachets as an adjunct to other drugs in the prevention and treatment of VAP. According to our study, when orodispersible probiotic sachets used in the diet of ICU patients, the cases of VAP were cut in half. Reduction in some complications of MV was also found. Therefore, we conclude that orodispersible probiotic sachets diet may be beneficial for the management of critically ill patients.
Authors' Contributions
Anand Prasoon was involved in concepts, design, definition of intellectual content, literature search, clinical studies, experimental studies, data acquisition, data analysis, manuscript preparation, and provided guarantee for this manuscript. Ritu Singh contributed to conceptualization, literature search, clinical studies, data acquisition, statistical analysis, manuscript preparation, editing and review, and provided guarantee for this manuscript. Ravi Anand contributed to conceptualization, design, literature search, clinical studies, experimental studies, data acquisition, statistical analysis, manuscript preparation, editing and review, and provided guarantee for this manuscript. Sanjeev Kumar was involved in definition of intellectual content, literature search, experimental studies, data analysis, and manuscript preparation, and provided guarantee for this manuscript.
Conflict of Interest
None declared.
References
- Effect of probiotics on incident ventilator-associated pneumonia in critically ill patients: a randomized clinical trial. JAMA. 2021;326(11):1024-1033.
- [Google Scholar]
- Do probiotics help prevent ventilator-associated pneumonia in critically ill patients? A systematic review with meta-analysis. ERJ Open Res. 2021;7(1):0302-2020.
- [Google Scholar]
- Efficacy of probiotics in the prevention of VAP in critically ill ICU patients: an updated systematic review and meta-analysis of randomized control trials. J Intensive Care. 2020;8:81.
- [CrossRef] [Google Scholar]
- International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur Respir J. 2017;50(3):50.
- [CrossRef] [Google Scholar]
- Management of ventilator-associated pneumonia: guidelines. Clin Chest Med. 2018;39(4):797-808.
- [Google Scholar]
- Challenges and opportunities in the treatment of ventilator-associated pneumonia. Expert Rev Anti Infect Ther. 2017;15(1):23-32.
- [Google Scholar]
- Disease burden of intensive care unit-acquired pneumonia in China: a systematic review and meta-analysis. Int J Infect Dis. 2014;29:84-90.
- [Google Scholar]
- Probiotics for preventing ventilator-associated pneumonia in mechanically ventilated patients: a meta-analysis with trial sequential analysis. Frontiers in.. 2017;9:8-717.
- [Google Scholar]
- Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the. Clin Infect Dis. 2016;63(5):e61-e111.
- [Google Scholar]
- Ventilator-associated events: prevalence, outcome, and relationship with ventilator-associated pneumonia. Crit Care Med. 2015;43(9):1798-1806.
- [Google Scholar]
- What's new in ventilator-associated pneumonia? Intensive Care Med. 2015;41(11):1954-1956.
- [Google Scholar]
- Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr Opin Pulm Med. 2013;19(3):216-228.
- [Google Scholar]
- Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13(8):665-671.
- [Google Scholar]







