Translate this page into:
Transesophageal Echocardiography (TEE)-Guided Paravalvular Leak (PVL) Closure: Expanding Horizons Beyond Operating Room
Poonam Malhotra Kapoor, MD, DNB, MNAMS, FISCU (Hony), FIACTA (Hony), FTEE (Hony) Department of Cardiac Anaesthesia and Critical Care, All India Institute of Medical Sciences New Delhi 110029 India drpoonamaiims@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Paravalvular leak (PVL) is a common, yet challenging entity occurring due to dehiscence of the annular tissue around the prosthetic valve, resulting in regurgitation of blood retrogradely. Although any prosthetic valve can be subjected to the risk of developing PVL, it is most commonly reported with the mitral valve followed by the aortic valve. The incidence of mitral PVL is around 7 to 17%, whereas with the aortic valve it is 5 to 10%. Symptomology can vary from asymptomatic patients with mild PVL to disabling symptoms pertaining to heart failure and hemolysis. TEE plays a pivotal role in the overall assessment along with procedural guidance for their closure. Multiple two-dimensional (2D) imaging views are required to scan the entire sewing ring diameter of a prosthetic valve. Three-dimensional (3D) TEE can give crucial information such as the number, size, shape, and circumference of the defects. 3D mitral en face view can give anatomical localization of the defect. During the procedure, TEE can assist in the confirmation of the position of the guidewire through the defect and not through the prosthetic valve. It also helps to conform to the adequate positioning of the vascular plug device and unrestrictive movement of the native prosthetic heart valve. TEE when combined with fluoroscopy can help in real-time guidance of passage of the guidewire and transcatheter device in relation to the prosthetic valve. Recently, Ahmed et al have named this technology as “Fusion Technique,” where they have combined real-time 2D and 3D TEE with fluoroscopy to facilitate the closure of PVL. Now that the time of minimally invasive surgery has taken over conventional surgery and fast tracking and enhanced recovery after surgery (ERAS) is the need of the moment, percutaneous PVL closure is preferred over surgical PVL closure. A study done by Gakrinho et al showed that percutaneous PVL closure has a reasonable success rate along with a low complication rate and the results are comparable to surgical treatment in high-risk patients. We hereby share our experience of the successful closure of PVL via the transcatheter technique using various 2D and 3D techniques.
Keywords
3D TEE
guided paravalvular leak closure
Percutaneous technique
Paravalvular leak (PVL) is a common, yet challenging entity occurring due to the dehiscence of the annular tissue around the prosthetic valve, resulting in regurgitation of blood retrogradely. Although any prosthetic valve can be subjected to the risk of developing PVL, it is most commonly reported with the mitral valve, followed by the aortic valve.1 The incidence of mitral PVL is around 7 to 17%, whereas with the aortic valve it is 5 to 10%.1, 2 Symptomology can vary from asymptomatic patients with mild PVL to disabling symptoms pertaining to heart failure and hemolysis.3
TEE was first applied in cardiac surgery in 1980.4 Earlier it was performed by combining a two-dimensional (2D) transducer on a fiber-optic endoscope. With the introduction of biplane and multiplane technology, TEE has increased its horizon from perioperative settings to cardiac cath labs in minimally invasive transcatheter technology. New advancement in three-dimensional (3D) TEE renders exact localization of the defect along with the size and direction of the jet. It helps in transseptal puncture and navigation of guidewire and occlusion devices through the defect under real-time guidance.
Fusion technique that combines 2D and 3D TEE along with fluoroscopy has resulted in improved anatomical detailing of PVL. We hereby share our experience of the successful closure of PVL via the transcatheter technique using various 2D and 3D techniques.
Case 1
A 35-year-old female patient, weighing 56 kg, who had undergone mitral valve replacement (#25 St.Jude mechanical valve) 6 years ago, had now come to the outpatient department with shortness of breath since 8 months. Dyspnea was gradually progressive in nature and now she is New York Heart Association (NYHA) class III. Blood investigations revealed a picture of hemolytic anemia with reduced hemoglobin, increased reticulocyte count, and raised unconjugated bilirubin. Transthoracic echocardiography revealed a PVL around the mitral prosthesis with mild left ventricular dysfunction (ejection fraction 45%). Once a diagnosis of PVL was made, the patient was scheduled for transcatheter paravalvular leak closure in the cath laboratory.
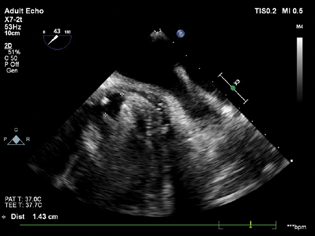
On the day of the surgery, the patient's NPO status was confirmed and routine ASA monitors were attached. Considering procedural complexity, duration along with a requirement of intraoperative TEE, general anesthesia with controlled ventilation was planned. After securing large bore IV access and invasive radial artery monitoring, the patient was induced with balanced anesthesia in the form of inj. midazolam 1 mg, Inj. fentanyl 100 µg, Inj. etomidate 14 mg. After confirmation of bag and mask ventilation, Inj. rocuronium 50 mg was given and the patient was intubated with an endotracheal tube (ETT) of 7.5 mm ID cuffed, fixed at the 21 mark. Once the ETT position was confirmed, the TEE probe was placed. The prosthetic mitral valve was viewed in a mid-esophageal (ME) 4-chamber, 2-chamber, and long axis view with special attention to the whole diameter of the swing ring (Fig. 1). Exact location, size, and direction of the PVL jet were noted. 3D TEE en face views were used to localize the size and extent of PVL leak (Fig. 2).

- Mid-esophageal long axis view showing paravalvular regurgitation.
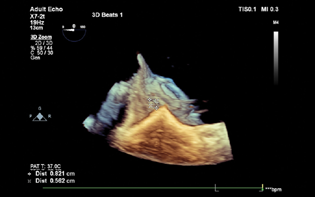
- 3D TEE showing exact site and dimension of the paravalvular leak.
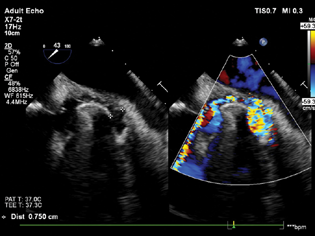
Antegrade trans-septal approach via the femoral vein was chosen for mitral PVL closure. The right femoral vein was cannulated with 8.5 Fr sheath under fluoroscopy guidance. Once the position was confirmed, a 14 Fr sheath was passed through an 8.5 Fr sheath. The patient was heparinized with 100 mg heparin to maintain a target ACT > 300. Mid-esophageal bicaval view was obtained to guide transeptal puncture (Fig. 3). A telescoping coaxial system was introduced into the LA, including transseptal LA sheath (8.5 French Agilis NXT Steerable introducer, St. Jude Medical, St. Paul, MN 55113), 100 CM 6 F coronary guide (multipurpose catheter), and a 5 Fr 125 cm multipurpose diagnostic catheter (Fig. 4). An exchange-length extra support angled hydrophilic 0.035 in wire (Terumo Medical Corp., Somerset, New Jersey, USA) was passed through the telescoping catheter system with a torque device to allow passage of the wire through the defect. 3D TEE was done for visualization of the wire through the defect (Fig. 5). Continuous fluoroscopy and TEE images helped in the successful insertion of the guide wire through the defect (Fig. 6). After crossing the defect, the wire was looped inside LV and traversed through the aortic valve into the descending thoracic aorta to maintain its position.
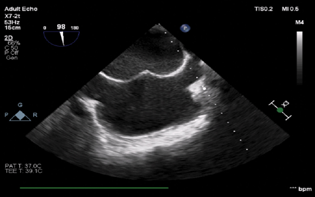
- Mid-esophageal bi-caval view showing trans septal puncture of the guide wire.
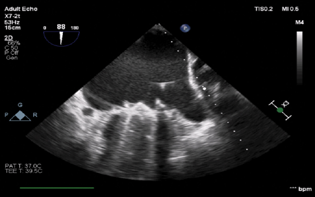
- Mid-esophageal two-chamber view demonstrating the guide wire in the left atrium.
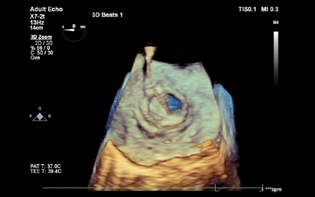
- 3D TEE image showing the position of the guide wire crossing through the prosthetic mitral valve.
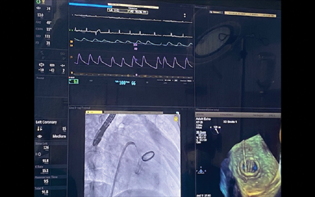
- Full assembly showing a simultaneous display of hemodynamic fluoroscopy as well as TEE imaging for successful delineation of the anatomy of the paravalvular leak.
A 5 Fr diagnostic catheter was crossed through the defect, which was followed by steady advancement of a 6 F coronary guide catheter into the LV. An Amplatzer vascular plug II device is railroaded over the 6 F coronary guide, and adequate position was confirmed by TEE. Immediate cessation of the paravalvular leak was noted (Figs. 7 and 8). Fluoroscopy revealed the normal movement of the mitral leaflet. The femoral sheath was removed once ACT < 150. The patient was shifted to the CCU and extubated after 2 hours.
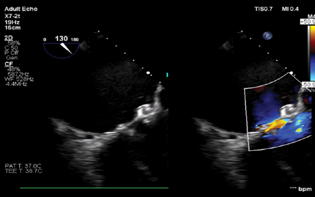
- Mid-esophageal long axis view showing successful closure of the paravalvular leak.
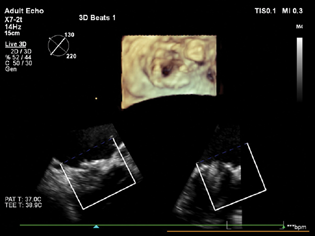
- Full-volume 3D images taken after the closure of the paravalvular leak, showing no residual paravalvular leak.
Case 2
A 45-year-old male patient weighing 60 kg had undergone aortic valve replacement (AVR) (#23 St. Jude mechanical valve) 5 years ago and presented with shortness of breath since 6 months. Dyspnea was initially on exertion but now it had progressed to daily household activity. He is now NYHA grade 3. Transthoracic echo revealed a PVL around the aortic valve with a normal ejection fraction (EF 50%). The patient was planned for transcatheter aortic valve closure.
Given the length and complexity of the procedure along with the requirement of TEE, the patient received general anesthesia with controlled ventilation. TEE was placed and the aortic valve was imaged in the ME aortic short axis and long axis view for site detection as well as quantification of the defect (Figs. 9–11). A retrograde approach via femoral artery access was chosen. A 6 Fr sheath was placed via the femoral artery. The periaortic defect was crossed with a 0.035 stiff-angled glide wire within a telescoped 125 cm 5 Fr multipurpose coronary catheter inside a 6 F multipurpose guiding catheter. An Amplatzer vascular plug was railroaded over the guidewire. TEE was done to guide the position of the vascular plug (Fig. 12). Normal movement of the prosthetic valve leaflet was noted. After the adequate reversal, the patient was extubated and shifted to the CCU uneventfully.
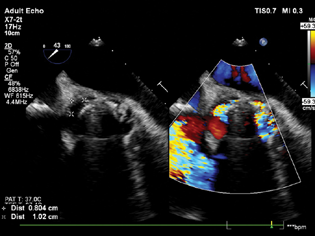
- Mid-esophageal aortic short axis view showing significant paravalvular leak around the aortic valve.
- Mid-esophageal aortic short axis view showing the size of the aortic paravalvular leak around the noncoronary cusp.
- Mid-esophageal aortic short axis view showing the size of the paravalvular leak around the left coronary cusp.
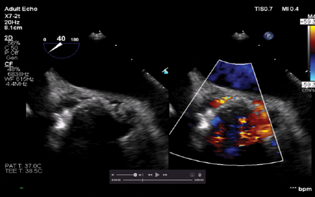
- Mid-esophageal aortic short axis view showing cessation of the paravalvular leak.
Discussion
Following prosthetic valve implantation, patients quite frequently develop PVL.1, 2 Risk factors associated with PVL are mitral position, supra-annular AVR, and continuous sutures in the mitral position, and use of unpledged sutures.5 PVL causes the volume and pressure overload to regurgitant chambers resulting in eccentric hypertrophy of the respective chamber. Back pressure changes occur in the pulmonary venous system, pulmonary artery, and subsequently to the right ventricle. Once the compensatory mechanism gets exhausted, the patient develops severe heart failure. Also, mechanical shear of red blood cells causes significant hemolysis. The development of moderate–severe PVL has been directly linked to increased mortality.6, 7 Surgical closure of PVL has been the traditional approach to patients; however, studies have reported that surgical repair has been associated with 30-day/in-hospital mortality of 8.5 to 11.5%.8, 9 Even after repeat surgery, 16 to 37% of patients had developed recurrent PVL.9, 10 In light of the above data, percutaneous catheter-based technique has been suggested as a minimally invasive strategy, which is suitable for even high-risk patients.11 Percutaneous repair of PVL was first utilized in 199212 and since then its application is ever increasing with the introduction of newer devices and improved 3D TEE and fluoroscopy technology. As far as an indication of percutaneous closure is concerned, all patients who are symptomatic with moderate to severe paravalvular regurgitation should undergo transcatheter closure. The American College of Cardiology/American Heart Association guidelines for the management of valvular heart disease recommends transcatheter closure as a class IIa indication for patients with intractable hemolysis or NYHA class III or IV heart failure, who have suitable anatomy for transcatheter closure and who are high-risk candidates for surgical closure.13 Absolute contraindications for percutaneous closure are patients having active endocarditis, with intracardiac thrombi and when regurgitant jet involves more than one-third of the circumference of the prosthetic annulus. TEE plays a pivotal role in the overall assessment along with procedural guidance for their closure. Multiple 2D imaging views are required to scan the entire sewing ring diameter of the prosthetic valve. 3D TEE can give crucial information such as the number, size, shape, and circumference of the defects. 3D mitral en face view can give anatomical localization of the defect. During the procedure, TEE can assist in the confirmation of the position of the guidewire through the defect and not through the prosthetic valve. It also helps to conform adequate positioning of the vascular plug device and unrestrictive movement of the native prosthetic heart valve. TEE, when combined with fluoroscopy, can help in real-time guidance of passage of the guidewire and transcatheter device passage in relation to the prosthetic valve. Recently, Ahmed et al have named this technology as “Fusion Technique,” where they have combined real-time 2D and 3D TEE with fluoroscopy to facilitate closure of PVL.14 Now that the time of minimally invasive surgery has taken over conventional surgery and fast tracking and enhanced recovery after surgery (ERAS) is the need of the moment, percutaneous PVL closure is preferred over surgical PVL closure. A study done by Gakrinho et al showed that percutaneous PVL closure has a reasonable success rate along with a low complication rate and the results are comparable to surgical treatment in high-risk patients.15
To conclude, percutaneous device closure is a useful approach in patients with moderate to severe PVL presenting with disabling symptoms, especially in the high-risk subset of patients who are not suitable candidates for surgical repair. Relevant history such as the type of prosthetic valve, number of prosthetic valves, its radiographic appearance, and exact anatomical localization of the jet should be known beforehand. Thorough knowledge of valvular anatomy and the ability to integrate real-time 3D images along with fluoroscopy are critical for the successful closure of PVL.
Conflict of Interest
None declared.
References
- Prevalence and clinical significance of incidental paraprosthetic valvar regurgitation: a prospective study using transoesophageal echocardiography. Heart. 2003;89(11):1316-1321.
- [Google Scholar]
- Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36(4):1152-1158.
- [Google Scholar]
- Tools and techniques–clinical: paravalvular leak closure. EuroIntervention. 2014;9(11):1359-1363.
- [Google Scholar]
- Importance of implant technique on risk of major paravalvular leak (PVL) after St. Jude mechanical heart valve replacement: a report from the Artificial Valve Endocarditis Reduction Trial (AVERT) Eur J Cardiothorac Surg. 2005;28(6):838-843.
- [Google Scholar]
- Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366(18):1686-1695.
- [Google Scholar]
- Impact of residual regurgitation after aortic valve replacement. Eur J Cardiothorac Surg. 2012;42(3):486-492.
- [Google Scholar]
- Surgical treatment of paravalvular leak: Long-term results in a single-center experience (up to 14 years) J Thorac Cardiovasc Surg. 2015;149(5):1270-1275.
- [Google Scholar]
- Long-term results of surgical correction for mitral paravalvular leak: repair versus re-replacement. J Heart Valve Dis. 2013;22(5):682-687.
- [Google Scholar]
- Early and late results of the surgical correction of cardiac prosthetic paravalvular leaks. J Heart Valve Dis. 2005;14(6):792-799. , discussion 799–800
- [Google Scholar]
- Long-term follow-up of percutaneous repair of paravalvular prosthetic regurgitation. J Am Coll Cardiol. 2011;58(21):2218-2224.
- [Google Scholar]
- Transcatheter umbrella closure of valvular and paravalvular leaks. J Am Coll Cardiol. 1992;20(6):1371-1377.
- [Google Scholar]
- 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):2438-2488.
- [Google Scholar]
- Paravalvular leak closure with real time transesophageal echocardiography and fluoroscopy fusion. JRSM Cardiovasc Dis. 2020;9:2048004020947290.
- [Google Scholar]
- Paravalvular leak closure: still a challenge with unpredictable results. Rev Port Cardiol (Engl Ed). 2021;40(4):261-269. (English Edition)
- [Google Scholar]






