Translate this page into:
Prospective Interventional Cohort Study using AIIMS Simplified POC Algorithm for Restricted Blood Transfusion in Cyanotic Children
*Corresponding author: Poonam Malhotra Kapoor, Department of Cardiac Anaesthesia and Critical Care, CNC, All India Institute of Medical Sciences, New Delhi, India. drpoonamaiims@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chakraborty S, Malhotra Kapoor P, Rajashekar P, Devagourou V, Patidar G, Mathiyalagen P. Prospective Interventional Cohort Study using AIIMS Simplified POC Algorithm for Restricted Blood Transfusion in Cyanotic Children. J Card Crit Care TSS. 2024;8:195-204. doi: 10.25259/JCCC_40_2024
Abstract
Objectives:
The coagulation parameters are known to be deranged in cyanotic congenital heart diseases (CCHDs). Perioperative hemorrhage, as well as massive blood and components transfusion, can cause hemodynamic and metabolic instability, which may lead to multi-organ damage and adversely affect the outcome of a well-performed surgical correction. In recent years, there are a number of studies shown that zero transfusion cardiac surgery, even with cardiopulmonary bypass (CPB), in adult as well as pediatric cardiac surgery is possible. This study yields a newer strategy to reduce over-ordering and transfusion of blood and blood products in cardiac surgeries, and in turn, this will also improve the outcome of cardiac surgeries, especially for CCHDs. Saved blood can save other lives, too.
Material and Methods:
Total 150 patients of either sex, with cyanotic congenital heart defect (CCHD) undergoing corrective surgery on CPB were used in this randomized controlled interventional cohort study. Written and informed consent was taken. Blood samples for ROTEM (Group A) and standard laboratory coagulation profile (Group B) were collected twice (T1: at aortic declaiming and T2: 10-15 minutes after protamine reversal). Blood and components were transfused according to ROTEM® algorithm or non POC algorithm (as per proposed algorithms) when indicated and outcomes recorded.
Results:
Despite a significant longer cardiopulmonary bypass time (140 vs. 128.5 min; P=0.019) in the POC group (ROTEM® based algorithm), we found a significant reduction in total drain output (357 vs. 426 mL; P=0.002), blood components transfusion (packed red blood cells, 3.0 vs. 6.0 U, P>0.001; plasma, 1 vs. 2 U, P<0.001; and platelets, 1 vs. 4 U, P<0.001), incidence of postoperative complications(42.5% vs. 65.7%, P=0.004), as well as duration of ICU stay (5 vs. 9 days, P=0.003). The reduction of in-hospital mortality was not significant (6.25% vs. 14.29%, P=0.102).
Conclusion:
ROTEM® based POC algorithm will guide us for rational blood components utilization and also help to reduce transfusion-related complications and duration of ICU stay.. Risk awareness and our easily interpretable algorithm will be helpful in this regard.
Keywords
Thromboelastometry
Prothrombin complex concentrate
Bleeding after cyanotic congenital heart disease surgery
Restricted blood transfusion
INTRODUCTION
Congenital heart diseases (CHDs) are developmental anomalies of the heart during intrauterine life. CHD may be acyanotic (ACHD) or cyanotic (CCHD).
CCHD patients are at high risk of peri- operative bleeding because of- reduced production of coagulation factors in view of hypoxic liver injury, deposition of platelets and coagulation factor in the sluggish vascular bed and also reduced platelet production due to erythrocytosis, relatively friable tissue, increased vascularity due to collaterals and release of nitric oxide (NO) from endothelium, hypothermia and also from effects of CPB. Peri-operative bleeding causes hypotension, metabolic acidosis and infection which further cause multi-organ damage.[1-4] CCHD have also been associated with abnormal coagulation, including low levels of fibrinogen, low platelet count and also platelet dysfunction,[5-7] making them susceptible to thrombosis and organ infarction.
Blood loss and coagulopathy is treated according to lab tests, like platelet count, prothrombin time (PT), activated partial thromboplastin time (aPTT), plasma fibrinogen concentration, with packed red blood cells (PRBC), fresh frozen plasma (FFP), platelet concentrate (PC), cryoprecipitate (CRYO) and hemostatic agents (like tranexamic acid, epsilon aminocaproic acid). However, due to relatively long reporting time and poor ability to predict exact cause of bleeding, there is high susceptibility of massive transfusion.
Blood transfusion is associated with various life-threatening complications, also, like transfusion related acute lung injury (TRALI), anaphylactic reaction, hemolytic and non-hemolytic reactions, transfusion related infections etc., resulting in increased morbidity, prolonged ventilator support, prolonged ICU stay and overall expenses. Judicious use of blood and blood components in cardiac surgery is hence very important.
Pediatric patients are not miniature adults because, the absolute circulating blood volume in them is much less than adults, thereby, even minor blood losses, which are insignificant in adults may be life threatening in pediatric age group.[8]
Reduction in the use of blood during cardiac surgery has started from the beginning of CPB yet to be achieved. As per recent study at least 30-70% of open-heart surgeries required blood transfusions.[9]
This study will guide us to formulate blood conservation strategies on the basis of our simplified point of care test algorithm to reduce the requirement of blood and blood products and their rational utilization. The development of this algorithm in easy-to-perform steps was the main aim of this research.
MATERIAL AND METHODS
Study design
This prospective randomized controlled trial with alternate allocation was conducted after Institutional Ethics Committee approval. Patients were enrolled after obtaining informed and written consent. None of them were on pre- operative anticoagulation therapy. Study was conducted as the following Consort Flow Diagram [Flowchart 1].
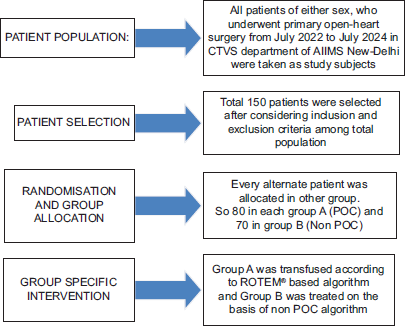
- Consort Flow Diagram.
- POC: Point of Care, ROTEM: Rotational Thromboelastometry, CTVS: Cardio Thoracic and Vascular Surgery.
Methodology
This randomized controlled trial was conducted in 150 patients. Patients were alternatively allocated with following inclusion criteria.
Inclusion criteria:
All congenital cyanotic heart disease patients undergoing corrective open heart surgery on CPB within the proposed study period were chosen.
Patients who have undergone first stage of palliation as BT shunt/PA Banding
Preserved ventricular function
Exclusion criteria:
Patients with known bleeding diathesis/hemoglobinopathies.
Patient with neurological abnormality preoperatively
Patient with gastrointestinal bleed
Reduced ventricular function
Redo surgeries involving CPB for second or third time in case of staged palliation
Patients not willing to give consent.
Pre anesthetic evaluation was done the night before surgery. Intra operatively patients were monitored for standard 5 leads ECG, invasive blood pressure, saturation (SPO2), central venous pressure (CVP), core temperature (nasopharyngeal), and urine output. First blood sample collection (T1) (2.7+2.7=5.4 ml), was taken at aortic declamping 30-45 minutes before weaning from CPB. 4 U/kg of unfractionated heparin (UFH) was given intra venously (IV) to all patients for a target activated clotting time (ACT) of >480 seconds.
Pre anesthetic evaluation was done the night before surgery. Intra operatively patients were monitored for standard 5 leads ECG, invasive blood pressure, saturation (SPO2), central venous pressure (CVP), core temperature (nasopharyngeal), and urine output. First blood sample collection (T1) (2.7+2.7=5.4 ml), was taken at aortic declamping 30-45 minutes before weaning from CPB. 4 U/kg of unfractionated heparin (UFH) was given intravenously (IV) to all patients for a target activated clotting time (ACT) of >480 seconds.
Sample collection
Blood samples for ROTEM® and coagulation profile tests were collected into 3.2% sodium citrate vacutainer for immediate analysis (after addition of calcification with 20µL of calcium chloride) and for hemoglobin and platelet count it was collected into spray dried ethylene diamine tetra acetate (EDTA)-k2 vacutainer and measured in a hematology analyzer.
Parameters evaluation
Patient demographic data (age, sex, height, weight,), pre op hematological test reports, peri-operative parameters (type of surgery, name of surgery, CPB details, number of blood and products required) were collected [Table 1].
| Parameters | Extem | Intem | Fibtem | Heptem And/Or Aptem |
|---|---|---|---|---|
| CT (seconds) | 3879 | 100240 | - | Better clot than INTEM=Heparin effect. |
| A10 (millimeter) | 4365 | 4466 | 723 | Better clot than EXTEM=Increased lysis. |
| MCF | 5072 | 5072 | 925 | Better clot than EXTEM=Increased lysis. |
ROTEM® parameters and standard lab parameters were noted. After shifting to ICU drain output and blood and components transfusion requirement, duration of ventilator support, inotropic support, duration of ICU stay and hospital stay were recorded.
Strategy used for blood conservation
General considerations
Pre-operative management
Restrictive transfusion strategy was preferred.
Multidisciplinary team (cardiac surgeon, cardiac anesthesiologists, ICU physician, transfusion medicine specialist, clinical pharmacist, perfusionist, and hematologist) discussion and planning regarding surgical strategy
Patients at high risk (advanced age, complex re-do surgery) of perioperative bleeding identification and proper planning
Pre-operative optimization of anemia, iron and coagulation profile, platelet count, and fibrinogen level. Administer pre-operative oral iron therapy or erythropoietin-stimulating agents along with oral iron supplementation several days before cardiac surgery to increase red cell mass
Platelet function testing should be done in patients taking anti-platelet therapy [Table 2].
| Physiological Transfusion Triggers | ||
|---|---|---|
| 1 | Hemoglobin (Hb) | Restrictive RBC transfusion threshold of Hb 7.5 g/dL is recommended in stable patients (TRICS trial), whereas patients undergoing highrisk cardiac surgery (ongoing ischaemia, CVA, history of CHF, microvascular bleeding), should be transfused to an Hb of 810 g/dL. RBCs has not been transfused if Hb ≥10 g/dL, with the potential exception of univentricular pediatric cardiac surgery patients. Hb level alone should not dictate transfusion but it should also be based on clinical factors such as rate of Hb decline, cardiopulmonary reserve, amount of acute blood loss being≥15% of total blood volume, etc. Recently collected RBCs (≤5 days old) should be used in children and neonates. |
| 2 | Haematocrit | A haematocrit of 24–30% to be kept during CPB when an adequate DO2 (>273 ml O2/min/m2) level is maintained. |
| 3 | Tranexamic acid or EACA Infusion | Tranexamic acid or EACA infusion significantly reduces allogeneic red blood cell transfusion. |
Intraoperative management
Auto-transfusion of shed mediastinal blood-Use of cell saver
Normothermic perfusion
Use of antifibrinolytics/blood components as indicated strict adherence to transfusion guidelines
Intraoperative hemofiltration (conventional ultrafiltration/modified ultrafiltration).
Strategy in the presence of point of care POC testing
In case of diffuse bleeding intraoperative (post-protamine) or significant bleeding (drain output (>2 mL/kg b.w./h) →first check and optimize body (core) temperature, blood pH, and ionized calcium level in ABG→Check ACT → if more than 150 s/or >20% of baseline ACT value and 20-50 for normal ROTEM parameters, chiefly/CTINTEM > 240; L130 (94 – 100%); FIBTEM MCF (9 - 25mm) and ML (<15%). If CTHEPTEM:CTINTEM<0.9 give additional protamine (0.5-0.8 mg/kg, never exceeding more than 1 mg/kg of protamine reversal) if act is less than baseline → go for other ROTEM® testing results to know the cause of bleeding.
In ROTEM® results, if CTEXTEM >100 s then → transfuse prothrombin complex concentrate (PCC) 10–15 iu/kg/(or) fresh frozen plasma (FFP) (10–15 mL/kg) (if PCC is not available) → If MLINTEM/EXTEM >7% of MCF (within 30 min)/>15% of MCF in 60 min → give tranexamic acid 15–25 mg/kg as bolus iv (in addition to local protocol) → if still bleeding not controlled and A10EXTEM ≤40 mm and A10FIBTEM <10 mm → transfuse CRYO (25 mL/unit) 1 unit/10 kg (or) fibrinogen concentrate with a target of A10FIBTEM >10–23 mm → but if A10EXTEM <40 mm and A10FIBTEM >9 mm then→patient needs platelet concentrate (PC) transfusion (50 mL/unit) of 1 unit/10 kg → If bleeding if still not controlled and still CTINTEM and CTHEPTEM >280 s (consider protamine overdose re-check CT after 10 min)→ consider additional FFP (10 mL/kg)→ if bleeding still continues surgical bleeding is most likely and surgical re-exploration and hemostasis. is needed. A5 FIBTEM has shorter turnaround time and similar diagnostic performance to A10 and can be used instead. New cyanotic POC All India Institute of Medical Sciences algorithm to implement blood conservation strategy is shown in Figure 1.
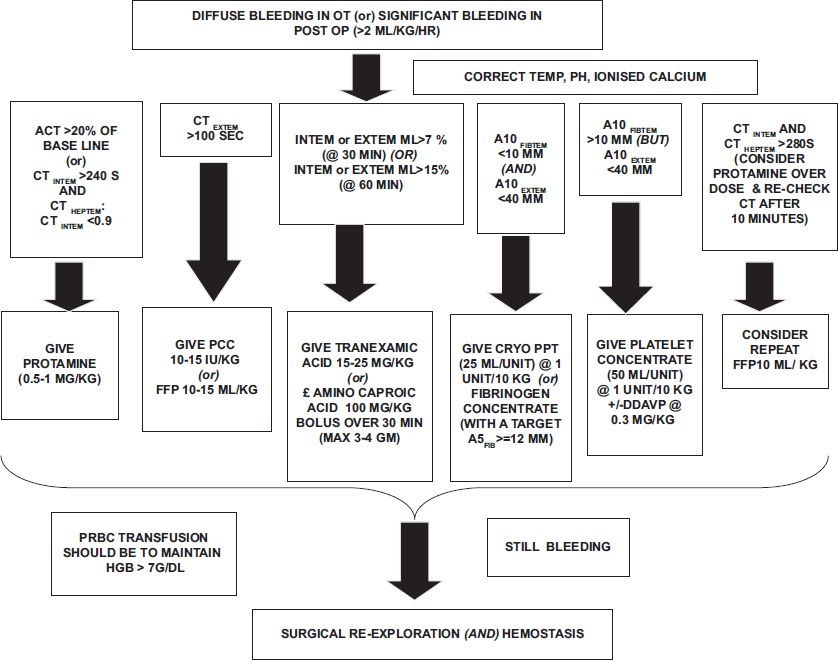
- New cyanotic POC All India Institute of Medical Sciences algorithm to implement blood conservation strategy. OT: Operation theater, ACT: Activated clotting time, CT: Clotting time, PCC: Prothrombin complex concentrate, FFP: Fresh frozen plasma, POC: Point of care, DDAVP: Desmopressin acetate.
Statistical analysis
Data was analyzed using SPSS version 20. KolmogorovSmirnov test was employed to assess the normality of the data. Pre-CPB (T1) and post-CPB (T2) POC parameters were compared using Mann-Whitney U test since the data followed non-normal distribution. Chi-square test or Fisher exact test was used to compare the categorical data. p value of <0.05 was considered statistically significant.
RESULTS
After analysis, we found mean aortic cross-clamp time in the POC group (group A) was 100.75 min and 90.20 min in the non-POC group (group B) (P = 0.351). The mean CPB time in group A was 158.10 min, and in group B, it was 131.03 min (P = 0.016). The mean total drain output (from surgery to removal) was 525.78 and 536.54 mL in group A and group B, respectively (P = 0.002). The mean duration of ventilator support was 37.86 and 71.60 h in group A and group B, respectively (P = 0.312), whereas the mean duration of ICU stay was 7.29 and 9.19 days in group A and group B, respectively (P = 0.003). Group A had a mean hospital stay of 13.78 days, whereas Group B had 14.41 days with P = 0.776. Postoperative complication rate in group A was 48.6% (34/70) and in group B 57.5% (46/80) (p value:0.275). Overall mortality rate in group A was 6.25% (5/80) and in group B was 14.29% (10/70) (p value:0.102) [see Tables 3, 8, and 9].
| Parameters (Mean Value) | Group A | Group B | P-Value |
|---|---|---|---|
| Clamp time (minutes) | 100.75±52.722 | 90.20±43.832 | 0.351 |
| CPB time (minutes) | 158.10±71.363 | 131.03±63.370 | 0.016 |
| Total drain output (ml) | 525.78±272.881 | 536.54±953.074 | 0.002 |
| Duration of ventilation (hours) | 37.86±26.54 | 71.61±113.42 | 0.312 |
| Duration of ICU stays (days) | 7.29±5.108 | 9.19±4.779 | 0.003 |
| Duration of hospital stay (post op days) | 13.78±9.150 | 14.41±9.505 | 0.776 |
| Post op complications* | 34/80 (42.5%) | 46/70 (65.71%) | 0.004 |
| Inhospital mortality* | 5/80 (6.25%) | 10/70 (14.29%) | 0.102 |
| Blood components (mean no of units transfused) | Group A | Group B | P-value |
|---|---|---|---|
| PRBC | 3.00 (3) | 6.00 (4) | <0.001 |
| FFP | 1.00 (2) | 2.00 (2) | <0.001 |
| PC | 1.00 (3) | 4.00 (4) | <0.001 |
| CRYO | 0.00 (1) | 0.00 (0) | 0.065 |
PRBC: Packed red blood cells, FFP: Fresh frozen plasma, CRYO: Cryoprecipitate, PC: Platelet concentrate
| Diagnosis | Group | Total (%) | |
|---|---|---|---|
| Non POC (%) | POC (%) | ||
| CCTGA | 5 (7.1) | 13 (16.2) | 18 (12.0) |
| CS TAPVC | 3 (4.3) | 0 (0.0) | 3 (2.0) |
| DTGA | 9 (12.9) | 12 (15.0) | 21 (14.0) |
| DORV | 4 (5.7) | 5 (6.2) | 9 (6.0) |
| TAPVC | 16 (22.9) | 17 (21.2) | 33 (22.0) |
| TGA IVS | 2 (2.9) | 1 (1.2) | 3 (2.0) |
| TOF | 27 (38.6) | 31 (38.8) | 58 (38.7) |
| TOF+PA | 4 (5.7) | 1 (1.2) | 5 (3.3) |
Chisquare test, Test statistic = 8.908;P = 0.259; Degree of freedom=7, POC: Point of care. CCTGA: Congenitally corrected transposition of great arteries, CS TAPVC: Coronary sinus totally anomalous pulmonary venous connection, D-TGA: Dextro-transposition of great arteries, DORV: Double outlet right ventricle, IVS: Intact ventricular septum, TOF: Tetralogy of Fallot, PA: Pulmonary atresia.
| Group | n | Mean±Standard Deviation | Median (IQR) | P-value | |
|---|---|---|---|---|---|
| Hb_LT1 | |||||
| NonPOC | 70 | 15.694±5.77 | 15.90 (6.9) | 0.230 | |
| POC | 80 | 18.254±8.77 | 16.30 (12.2) | ||
| HCT_LT1 | |||||
| NonPOC | 70 | 48.423±14.86 | 49.15 (21.4) | 0.245 | |
| POC | 80 | 51.300±52.86 | 44.35 (23.8) | ||
| TLC_LT1 | |||||
| NonPOC | 70 | 14.2413±5.25 | 13.34 (10.6) | 0.776 | |
| POC | 80 | 13.8111±4.95 | 13.45 (8.19) | ||
| Platelet (Lakh)_LT1 | |||||
| NonPOC | 70 | 2.6463±1.13 | 2.36 (1.12) | 0.340 | |
| POC | 80 | 2.9244±1.44 | 2.59 (1.86) | ||
| PT_LT1 | |||||
| NonPOC | 68 | 17.142±3.63 | 17.35 (4.4) | <0.001 | |
| POC | 80 | 14.954±3.65 | 14.85 (6.3) | ||
| INR_LT1 | |||||
| NonPOC | 68 | 1.41303±0.28 | 1.29 (0.45) | 0.290 | |
| POC | 80 | 1.47862±0.36 | 1.36 (0.58) | ||
| APTT_LT1 | |||||
| NonPOC | 67 | 30.203±5.81 | 30.00 (4.0) | 0.049 | |
| POC | 80 | 31.704±5.76 | 32.00 (7.8) | ||
| ACT (S)_LT1 | |||||
| NonPOC | 70 | 162.13±58.61 | 148.50 (57) | 0.057 | |
| POC | 80 | 185.70±74.88 | 176.00 (107) | ||
Mann–Whitney Utest was applied. APTT: Activated partial thromboplastin time, IQR: Interquartile range, ACT: Activated clotting time, PT: Prothrombin time, INR: International normalized ratio, Hb: Hemoglobin, HCT: hematocrit, TLC: Total lymphocyte count, POC: Point of care. P value: <0.05=Significant.
| Group (N1_P2) | n | Mean±Standard Deviation | Median (IQR) | P-value | |
|---|---|---|---|---|---|
| PRBCREQ | |||||
| NonPOC | 70 | 8.37±1.746 | 8.00 (2) | <0.001 | |
| POC | 80 | 4.08±1.028 | 4.00 (1) | ||
| PRBCTRANS | |||||
| NonPOC | 70 | 6.16±3.072 | 6.00 (4) | <0.001 | |
| POC | 80 | 3.05±1.735 | 3.00 (3) | ||
| FFPREQ | |||||
| NonPOC | 70 | 5.86±3.089 | 4.00 (2) | <0.001 | |
| POC | 80 | 3.76±1.989 | 3.00 (4) | ||
| FFPTRANS | |||||
| NonPOC | 70 | 3.43±2.790 | 2.00 (2) | <0.001 | |
| POC | 80 | 0.86±0.910 | 1.00 (2) | ||
| PCREQ | |||||
| NonPOC | 70 | 6.34±4.851 | 4.00 (2) | <0.001 | |
| POC | 80 | 3.01±1.804 | 3.00 (1) | ||
| PCTRANS | |||||
| NonPOC | 70 | 4.96±5.470 | 4.00 (4) | <0.001 | |
| POC | 80 | 1.66±1.896 | 1.00 (3) | ||
| CRYOREQ | |||||
| NonPOC | 70 | 2.11±3.420 | 0.00 (4) | 0.874 | |
| POC | 80 | 1.15±1.647 | 1.00 (2) | ||
| CRYOTRANS | |||||
| NonPOC | 70 | 1.03±3.022 | 0.00 (0) | 0.065 | |
| POC | 80 | 0.76±1.495 | 0.00 (1) | ||
Mann–Whitney Utest was applied. PRBC: Packed red blood cells, FFP: Fresh frozen plasma, CRYO: Cryoprecipitate, PC: Platelet concentrate, IQR: Interquartile range, POC: Point of care. P value: <0.05=Significant.
| Post-op Complications | With Complications | Without Complications | Overall |
|---|---|---|---|
| Group A | 34/80 (42.5%) | 46/80 (57.5%) | 80 (100%) |
| Group B | 46/70 (65.71%) | 24/70 (34.29%) | 70 (100%) |
| Group A+B | 80/150 (53.33%) | 70/150 (46.67%) | 150 (100%) |
Risk ratio, RR [95% CI] = 0.6467 [0.4763 to 0.8782]; Z=2.792; P=0.0052; NNT (Benefit) [95% CI] = 4.308 [2.579 (Benefit) to 13.075 (Benefit)]. The RR of 0.6467 suggests that the POC-group A had a 35.3% lower risk of complications compared to the Non-POC-group B. The 95% confidence interval (0.4763 to 0.8782) does not include 1, indicating that this result is statistically significant. Therefore, POC group appears to be associated with a reduced risk of post op complications. The number needed to treat (NNT) of 4.3 means that 4.3 patients have to be treated according to the POC-algorithm to avoid complications in one patient.
| In-Hospital Mortality | Died | Survived | Overall |
|---|---|---|---|
| Group A | 5/80 (6.25%) | 75/80 (93.75%) | 80 (100%) |
| Group B | 10/70 (14.29%) | 60/70 (85.71%) | 70 (100%) |
| Group A+B | 15/150 (10%) | 135/150 (90%) | 150 (100%) |
Risk ratio, RR [95% CI] = 0.4375 [0.1571 to 1.2187]; Z=1.582; P=0.1138; NNT (Benefit) [95% CI] = 12.444 [66.605 (Harm) to∞to 5.691 (Benefit)]. The risk ratio of 0.4375 indicates that the POC group had a 56.3% lower risk of mortality compared to the Non-POC group. However, the 95% confidence interval (0.1571 to 1.2187) includes 1, suggesting that this result is not statistically significant. The number needed to treat (NNT) of 12.4 means that 12.4 patients have to be treated according to the POC-algorithm to save one life.
Regarding transfusion requirement, group A required an average of 3.05 units of PRBC, 0.86 units of FFP, 1.66 units of PC, and 0.76 units of CRYO transfusion, whereas group B required 6.16 units of PRBC, 3.43 units of FFP, 4.96 units of PC, and 1.03 units of CRYO transfusion. Except for CRYO (P < 0.065), all other components transfusion was significantly lower in group A (P < 0.001) in our study [Table 4].
There was no statistically significant difference between the two groups with respect to diagnosis [Table 5].
There was a statistically significant difference between the two groups with respect to PT and APTT [Table 6 and Figure 2].
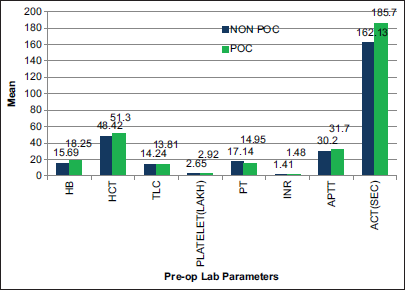
- Pre-operative laboratory parameters (T1) difference between two groups. APTT: Activated partial thromboplastin time, ACT: Activated clotting time, PT: Prothrombin time, INR: International normalized ratio, Hb: Hemoglobin, HCT: hematocrit, TLC: Total lymphocyte count,POC: Point of Care.
There was a statistically significant difference between the two groups with respect to PRBC requested, PRBC transfused, FFP requested, FFP transfused, PC requested, and PC transfused [Table 7].
DISCUSSION
Congenital heart disease (CHD) prevalence is about 8–9/1000 live births, with approximately 25% are cyanotic congenital heart diseases. Among them, tetralogy of Fallot accounts for 5%, and transposition of great arteries accounts for 2% of cases and presents in the first week of life.[11]
In a hospital, the majority of blood transfusions are for cardiac surgical patients. Leukocyte-related target organ damage (2–5%), febrile non-hemolytic transfusion reaction (1%), transfusion-related lung injury (0.05%), and bacterial infection/sepsis (0.05%) are the most common complications of blood transfusion.[12] Risk of cardiac complications such as – low cardiac output syndrome, increased inotropic usage, acute kidney injury, duration of mechanical ventilation, longer intensive care unit (ICU) and hospital stay, and overall, increased treatment cost, morbidity, and mortality are also increased with more transfusion of blood and blood products.[13-18]
The number of complex congenital heart surgeries is increasing nowadays. In children, the average amount of perioperative blood loss and blood transfusion are up to 15–110 mL/kg and 155 mL/kg, respectively.[19,20] Chambers et al., in 1996, showed that 98% of children undergoing on-pump cardiac surgery required blood transfusion,[21] while recent reports tell us that 38–74% of children undergoing cardiac surgery required blood and blood products transfusion.[22]
Children are at high risk of hemodilution and coagulopathies from CPB priming solutions, intravenous fluids, inability to maintain the cardiac output on demand and operative bleeding.[23]
Minimized circuit volume, ultrafiltration, anti-fibrinolytics use, and proper treatment of coagulopathy can limit the transfusion requirements. Various factors such as patient age and weight, surgical complexity, duration of surgery, degree of cyanosis, and CPB-induced coagulopathies can influence anemia tolerance, risk of bleeding, and transfusion requirement.[17,23-25]
According to a study of 2006 on “morbidity and mortality risk associated with red blood cell (RBC) and blood-component transfusion,” they found that, following cardiac surgery without and with PRBCs transfusion, renal morbid events were 0% and 1. 81%; prolonged ventilator support, 0.44% and 9.14%; serious post-operative infection, 0.24% and 5.03%; cardiac morbidity, 0.05% and 3.03%; neurologic morbidity, 0.37% and 2.41%; and overall post-operative morbidity, 0.96% and 12.33%, respectively, and overall mortality for patients without transfusion was 0. 05%, while, in PRBC transfusion group, it was 3.07%.[25,26]
This was more helpful in predicting the need for haemostatic interventions after heparin reversal by protamine. The second blood sample (T2) taken at 10-15 min after protamine in bleeding patients? Late blood sampling in bleeding patients results in a delay in treatment particularly with interventions that are not immediately available such as cryoprecipitate and further blood loss.
Late blood sampling in bleeding patients result in a delay in treatment interventions particularly with which are not immediately available such as cryoprecipitate and further blood loss.
Since PRBC transfusion may be the primary endpoint of this study (unfortunately not specified in the method section), the transfusion trigger and target for PRBC transfusion is very important. Secondary endpoints are mortality. complication rate, patient outcome and length of ICU stay.
According to the combined results of two National Institute of Health-supported trials in 2008, the goal HCT of 30 or 35% could be achieved during CPB without transfusion. These authors also concluded that an HCT >24% during CPB has better psychomotor development at 1 year of age.[26]
Red blood cells (RBC) transfusion improves oxygen delivery, so the main rationale for RBC transfusion in pediatric cardiac surgery patients is to treat anemia.
Multidisciplinary approach including use of low-prime volume circuits with or without vacuum-assisted venous drainage, ultra-filtration (pre-bypass, conventional, and modified), and “cell-saver” for all pediatric patients.[28-30]
Restrictive transfusion strategy has a similar outcome in comparison to a liberal transfusion strategy for adult cardiac patients in terms of all-cause mortality, myocardial infarction, stroke, or new-onset renal failure with dialysis on 28 days to 6 months after surgery.[31]
Common causes of mortality in patients of post-CCHD surgery are – age at the time of operation, complexity of surgery, massive transfusion, and prolonged bypass time.[14,15] It is now well-accepted that perioperative blood transfusion is a modifiable risk factor causing mortality and morbidity in children undergoing congenital cardiac surgery[15,16]
ROTEM-guided patient blood management (PBM) has been shown to be effective in reducing bleeding, transfusion requirements, complication rates, and healthcare costs.[32]
Recent PBM strategy published by European Association for Cardio Thoracic Surgery (EACTS) and European Association of Cardiothoracic anesthesia (EACTA) provides practical recommendations for adult cardiac surgeries[33] but specific guidelines for pediatric cardiac surgery are still lacking. Use of POC tests will help in transfusing right blood components to reduce post-operative bleeding in cyanotic children also[34] which in turn restricts the exposure to blood and blood components and was associated with reduced ICU and hospital stay in pediatric congenital cyanotic surgical patients.[35-44]
In our study also, in spite of relatively higher CPB and cross-clamp time in group A patients, we found statistically significant lower drain output, a reduced incidence of postoperative complications and a shorter duration of the ICU stay [Tables 1,3,8,9]. The reduction of in-hospital mortality from 14.29 to 6.25% (RR = 0.4375; P = 0.1138) did not reach statistical significance; however, the study was not powered for a difference in mortality. All the blood component transfusion requirements were significantly lower in group A (where we followed POC algorithm), too, except for cryoprecipitate [Table 2].
In summary, despite a longer CPB time in group A – which is a known good predictor for bleeding, transfusion and bad outcome – we found a significant lower total drain output, transfusion requirements, post op complication rate, and ICU length of stay in the POC-guided group A [Tables 3,4,8, and 9]. However, the reduction in mortality did not reach statistical significance (Risk ratio [95% Confidence Interval]; = 0.4375 [0.1571 to 1.2187]; p = 0.1138) but the study was not powered for a difference in mortality. Furthermore, all blood components transfusion requirements were significantly lower in group A (POC-guided group), except for cryoprecipitate which did not change significantly [Table 4].
Despite satisfactory results, our study has some limitations such as being a single center, single surgeon study with limited patient number. This algorithm may not be cost effective in a developing country like ours. Relatively high infection rate and sepsis being the major cause of complication and mortality, coagulation profile may also be deranged in such cases. There was no long-term follow-up data taken into consideration in this study.
CONCLUSION
Our ROTEM® based POC algorithm is a good guide for rational and restricted blood components utilization and may helps to avoid transfusion related complications too. Risk awareness and our easily interpretable algorithm will be helpful in the intra and post-operative care of pediatric cyanotic congenital cardiac surgical patients.
Ethical approval
The research/study approved by the Institutional Review Board at All India Institute of Medical Sciences (AIIMS), number IECPG-451/June 30, 2022, RT 37/July 28, 2022, dated July 29, 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Poonam Malhotra Kapoor is on the editorial board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Increased Mortality, Postoperative Morbidity, and Cost after Red Blood Cell Transfusion in Patients Having Cardiac Surgery. Circulation. 2007;116:2544-52.
- [CrossRef] [Google Scholar]
- Active Bleeding after Cardiac Surgery: A Prospective Observational Multicenter Study. PLoS One. 2016;11:e0162396.
- [CrossRef] [Google Scholar]
- Mitigating the Risk: Transfusion or Reoperation for Bleeding after Cardiac Surgery. Ann Thorac Surg. 2019;14:124-9.
- [Google Scholar]
- Coagulation Abnormalities in Patients with Cyanotic Congenital Heart Disease. J Cardiothorac Vasc Anesth. 2002;16:752-65.
- [CrossRef] [Google Scholar]
- The Effect of Cyanosis on Perioperative Platelet Function as Measured by Multiple Electrode Aggregometry and Postoperative Blood Loss in Neonates and Infants Undergoing Cardiac Surgery. Eur J Cardiothorac Surg. 2015;48:301-7.
- [CrossRef] [Google Scholar]
- Haematological Considerations in Patients with Cyanotic Congenital Heart Disease: A Review. Dent Update. 2006;33(617-8):620-2.
- [CrossRef] [Google Scholar]
- Fibrinogen Function is Impaired in Whole Blood from Patients with Cyanotic Congenital Heart Disease. Int J Cardiol. 2013;167:2210-4.
- [CrossRef] [Google Scholar]
- Preparation and Use of Resuscitation Equipment to Assess and Treat Children in Emergency Situations. Nurs Child Young People. 2015;27:30-7.
- [CrossRef] [Google Scholar]
- Multi-Modality Blood Conservation Strategy in Open-heart Surgery: An Audit. Interact Cardiovasc Thorac Surg. 2009;9:480-2.
- [CrossRef] [Google Scholar]
- Multi-centre Investigation on Reference Ranges for ROTEM Thromboelastometry. Blood Coagul Fibrinolysis. 2005;16:301-10.
- [CrossRef] [Google Scholar]
- Cyanotic Heart Disease In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500001 [Last accessed on 2021 Nov 07]
- [Google Scholar]
- Challenges and Progress of the Pediatric Cardiac Surgery in Shanghai Children's Medical Center: A 25-year Solid Collaboration with Project HOPE. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2009;12:12-8.
- [CrossRef] [Google Scholar]
- Risk Factors Prolonging Ventilation in Young Children after Cardiac Surgery: Impact of Noninfectious Pulmonary Complications. Pediatr Crit Care Med. 2002;3:269-74.
- [CrossRef] [Google Scholar]
- Association of Complications with Blood Transfusions in Pediatric Cardiac Surgery Patients. Ann Thorac Surg. 2013;96:910-6.
- [CrossRef] [Google Scholar]
- Blood Transfusion is Associated with Prolonged Duration of Mechanical Ventilation in Infants Undergoing Reparative Cardiac Surgery. Pediatr Crit Care Med. 2011;12:52-6.
- [CrossRef] [Google Scholar]
- Risks and Predictors of Blood Transfusion in Pediatric Patients Undergoing Open Heart Operations. Ann Thorac Surg. 2009;87:187-97.
- [CrossRef] [Google Scholar]
- Morbidity and Mortality Risk Associated with Red Blood Cell and Blood-component Transfusion in Isolated Coronary Artery Bypass Grafting. Crit Care Med. 2006;34:1608-16.
- [CrossRef] [Google Scholar]
- Six-Month Outcomes after Restrictive or Liberal Transfusion for Cardiac Surgery. N Engl J Med. 2018;379:1224-33.
- [CrossRef] [Google Scholar]
- Comparison of the Hemostatic Effects of Fresh Whole Blood, Stored Whole Blood, and Components after Open Heart Surgery in Children. Blood. 1991;77:930-6.
- [CrossRef] [Google Scholar]
- Aprotinin in Pediatric Cardiac Operations: Platelet Function, Blood Loss, and Use of Homologous Blood. Ann Thorac Surg. 1993;55:1460-6.
- [CrossRef] [Google Scholar]
- Transfusion Patterns in Pediatric Open Heart Surgery. Transfusion. 1996;36:150-4.
- [CrossRef] [Google Scholar]
- Anemia and Red Blood Cell Transfusion in Critically Ill Cardiac Patients. Ann Intensive Care. 2014;4:16.
- [CrossRef] [Google Scholar]
- Patient Blood Management in Pediatric Cardiac Surgery: A Review. Anesth Analg. 2018;127:1002-16.
- [CrossRef] [Google Scholar]
- Nadir Hematocrit During Cardiopulmonary Bypass: End-organ Dysfunction and Mortality. J Thorac Cardiovasc Surg. 2012;144:654-62. e4
- [CrossRef] [Google Scholar]
- Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608-16.
- [CrossRef] [Google Scholar]
- The Effect of Hematocrit during Hypothermic Cardiopulmonary Bypass in Infant Heart Surgery: Results from the Combined Boston Hematocrit Trials. J Thorac Cardiovasc Surg. 2008;135:355-60.
- [CrossRef] [Google Scholar]
- Blood Transfusion is Associated with Prolonged Duration of Mechanical Ventilation in Infants Undergoing Reparative Cardiac Surgery. Pediatr Crit Care Med. 2011;12:52.
- [CrossRef] [Google Scholar]
- Minimizing the Need for Transfusion in Pediatric Congenital Heart Surgery. Int J Clin Transfus Med. 2019;7:1-9.
- [CrossRef] [Google Scholar]
- Pediatric Acute Lung Injury and Sepsis Investigators Network. Blood Transfusions after Pediatric Cardiac Operations: A North American Multicenter Prospective Study. Ann Thorac Surg. 2015;100:671-7.
- [CrossRef] [Google Scholar]
- Anemia, Blood Loss, and Blood Transfusions in North American Children in the Intensive Care Unit. Am J Respir Crit Care Med. 2008;178:26-33.
- [CrossRef] [Google Scholar]
- Restrictive or Liberal Red-cell Transfusion for Cardiac Surgery. N Engl J Med. 2018;377:2133-44.
- [CrossRef] [Google Scholar]
- The Role of Evidence-Based Algorithms for Rotational Thromboelastometry-Guided Bleeding Management. Korean J Anesthesiol. 2019;72:297-322.
- [CrossRef] [Google Scholar]
- 2017 EACTS/EACTA Guidelines on Patient Blood Management for Adult Cardiac Surgery. J Cardiothorac Vasc Anesth. 2018;32:88-120.
- [CrossRef] [Google Scholar]
- Congenital Cyanotic Cardiac Surgery in Children: Is Algorithm-Based Point-of-Care Testing Essential to Prevent Bleeding? J Cardiac Crit Care TSS. 2018;2:84-90.
- [CrossRef] [Google Scholar]
- Massive Transfusion/Hemorrhage Protocols Versus Goal-Directed Bleeding Management: Science Gone Eerie? J Cardiac Crit Care TSS. 2024;8:16-27.
- [CrossRef] [Google Scholar]
- Is Rotational Thromboelastometry the Answer for Rapid Prediction of Coagulopathy on Extracorporeal Membrane Oxygenation? J Cardiac Crit Care TSS. 2017;1:108-10.
- [CrossRef] [Google Scholar]
- A Prospective Randomized Clinical Trial of Efficacy of Algorithm-Based Point of Care Guided Hemostatic Therapy in Cyanotic Congenital Heart Disease Surgical Patients. J Cardiac Crit Care TSS. 2019;3:8-16.
- [CrossRef] [Google Scholar]
- Platelet Function Test in Coronary Artery Bypass Grafting: Does It Predict Postoperative Bleeding? J Cardiac Crit Care TSS. 2021;5:186-95.
- [CrossRef] [Google Scholar]
- Role of Platelet Function Test in Predicting Postoperative Bleeding Risk after Coronary Artery Bypass Grafting: A Prospective Observational Study. J Cardiac Crit Care TSS. 2021;5:88-96.
- [CrossRef] [Google Scholar]
- Utility of Platelet Function Tests: A Recent Review Round Up. J Card Crit Care TSS. 2020;3:24-7.
- [CrossRef] [Google Scholar]
- Thromboelastography in Venovenous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. J Card Crit Care TSS. 2017;1:101-4.
- [CrossRef] [Google Scholar]
- Anticoagulation During ECMO: Will the Tight Rope be Tighter in 2018? J Card Crit Care TSS. 2017;1:55-6.
- [CrossRef] [Google Scholar]
- Patient Blood Management: Moving Above and Beyond the Optimal Use of Blood! J Card Crit Care TSS. 2024;8:28-32.
- [CrossRef] [Google Scholar]
- Implementation of Patient Blood Management-A Long and Winding Road but Worth Doing! J Card Crit Care TSS. 2024;8:1-4.
- [CrossRef] [Google Scholar]







