Translate this page into:
Hematological Profile of Congenital Heart Disease Patients undergoing Surgical Correction: A Case–control Observational Study from North India
*Corresponding author: Prachi Kukshal, Department of Genetics, Sri Sathya Sai Sanjeevani Research Foundation, Palwal, Haryana, India. prachi7k@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ahamad S, Kukshal P, Kumar A, Tulsi A, Sharma A, Singh P. Hematological Profile of Congenital Heart Disease Patients undergoing Surgical Correction: A Case–control Observational Study from North India. J Card Crit Care TSS. 2024;8:205-16. doi: 10.25259/JCCC_34_2024
Abstract
Objectives:
Congenital heart disease (CHD) is a cardiac birth anomaly, contributing to ~28% of all birth defects, causing higher fetal and neonatal mortality worldwide. Complete blood count (CBC) is a widely used test for clinical investigation of the patient and is reported to predict the risk of cardiovascular disease and other metabolic disorders. This study reports the correlation between CBC indices and CHD.
Material and Methods:
n = 238 CHD patients and 50 healthy controls were enrolled. CBC was done with pre-operative blood. Student’s t-test, Chi-square test, and multivariate logistic regression were performed for statistical analysis.
Results:
~79% (11 out of 14) parameters showed significant deviation from the controls. Lymphocytosis and low platelet-to-lymphocyte ratio were prominently demonstrated in all cases (P = 0.000), along with erythrocytosis in the cyanotic group. Interestingly, cyanotic patients, wherein anemia is more common, had significantly higher hemoglobin (HGB) (P = 0.000). Multivariate regression showed a strong correlation of hematocrit (HCT) with HGB (r = 0.92) and oxygen saturation (SpO2) (r = −0.76), red blood cell with HCT (r = 0.88), HGB (r = 0.83), and SpO2 (r = −0.78). Higher pulse, platelet counts and lymphocytes, low body mass index, mean corpuscular volume, HGB, and mean corpuscular hemoglobin may result in early diagnosis (P < 0.05) while decreased mean corpuscular hemoglobin concentration level can reduce ventilation time (P = 0.0004).
Conclusion:
Our study highlighted the relationship between CBC and CHD and their impact on the hospitalization status of patients from the North Indian cohort.
Keywords
Congenital heart disease
Hematology
Association
North India
INTRODUCTION
Congenital heart disease (CHD) is the most common cardiac birth defect, causing maternal, fetal, and neonatal morbidity and mortality.[1-3] According to the “Global Report of Birth Defects” (2006), around 7.9 million children are born with birth defects globally; among them 1.7 million are only CHD cases, causing 0.25 million deaths worldwide.[4,5] Its prevalence in Asia is 9.3/1000 live births and varies from 1.3 to 9.2/1000 in the Indian population. The prevalence is comparatively high in the Northern and Eastern regions of India due to high birth rates.[2] Around 20–30% of CHD manifestation is accountable to genetic and non-genetic (environmental) factors, while ~60% of causes are unknown.[6]
Complete blood count (CBC) analysis can effectively reveal complex changes in inflammatory activation, which helps in predicting the prognosis of cardiovascular diseases hence enabling the adverse outcomes following cardiac surgery. Due to the simplicity, reproducibility, and readily availability of results, CBC is widely used for clinical examination of the patient. Abnormal leukocyte number and alterations in its subpopulation counts could be indicative of immune response to more complicated inflammatory indices, such as neutrophilto-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR) and can be used to predict cardiovascular and several non-cardiac defects.[7] Lymphocyte count indicates the immune regulatory responses, physiological stress, and degree of inflammation.[8] Platelets (PLT) are a part of the body’s inflammatory action and secrete certain chemokines, cytokines, and growth factors to maintain homeostasis, and they have been shown to exhibit both qualitative and quantitative malformations in cyanotic CHD.[9,10]
Unbalancing of hematological parameters such as neutropenia and neutrophilia, lymphopenia (<600 cells/L) and lymphocytosis (>4500 cells/L), thrombocytopenia and thrombocytosis, etc., may result in a longer intensive care unit (ICU) and hospital stay, increased ventilation period, post-operative infection, and sepsis, pulmonary and arterial hypertension, complicated surgical procedure, heart failure, and sometimes mortality.[7] Early detection and timely management of the causal factors of these abnormal parameters may play a significant role in improving surgical outcomes and minimizing post-operative complications. Previous studies have shown a strong association of deviated hematological indices with coronary heart defect and other cardiovascular diseases, but very limited studies and literature are available that demonstrate the association of these parameters with CHD.[11] This study aimed to find a correlation of CBC parameters with CHD and contribute to giving insight into these blood biomarkers for the prognostic evaluation of the disease.
MATERIAL AND METHODS
Study design
This case–control observational study was conducted on the retrospective pre-operative laboratory data of patients of north Indian origin who underwent congenital cardiac interventions after echocardiography confirmation from June to December 2023 at Sri Sathya Sai Sanjeevani International Hospital Palwal (India), a totally free-of-cost tertiary pediatric cardiac care center. The Institutional Ethics Committee approval and written informed consent/assent were obtained at DSIR-SIRO-certified Sri Sathya Sai Sanjeevani Research Foundation. n = 238 CHD cases, including 191 (80.25%) acyanotic and 47 (19.75%) cyanotic cases, were recruited. These categories were further divided into ventricular septal defects (VSD) (n = 73), atrial septal defects (ASD) (n = 58), tetralogy of fallot (TOF) (n = 35), patent ductus arteriosus (PDA) (n = 40), and miscellaneous (n = 32) [Supplementary Table 1]. Patients who had a recent blood/platelet transfusion, iron supplementation, syndromic features, extracardiac anomalies, or any other chronic/hematological disorder based on clinical history were excluded. The CBC was performed using a Swelab Alfa Hematology analyzer (Boule, Sweden). The vitals were captured before blood collection. n = 50 healthy controls, after an echocardiography confirmation and written consent of the same ethnicity and inclusion criteria, were included in the association study [Table 1].
| Variable | Controls (n=50) | Cases (n=238) | Acyanotic (n=191) | Cyanotic (n=47) | P-values (w.r.t. control) | P-value | ||
|---|---|---|---|---|---|---|---|---|
| All Cases | Acyn | Cyn | Acyn vs. Cyn | |||||
| Gender | ||||||||
| Male | 28 | 145 | 110 | 35 | 0.52 | 0.84 | 0.06 | 0.03 |
| Female | 22 | 93 | 81 | 12 | ||||
| Age (Y) | 22.5 (0.11–68) | 5 (0.06–44.5) | - | - | - | - | - | - |
| BMI (kg/m2) | 21.99±0.69 | 13.54±0.17 | 13.44±2.22 | 13.96±3.66 | 0.000 | 0.000 | 0.000 | 0.34 |
| Blood group | ||||||||
| A | 13 | 62 | 50 | 12 | 0.76* | 0.65* | 0.97* | 0.69* |
| B | 15 | 90 | 71 | 19 | 0.93* | 0.45* | 0.35* | 0.055* |
| AB | 6 | 13 | 10 | 3 | 0.56* | 0.69* | 0.71* | 0.95* |
| O | 16 | 67 | 54 | 13 | 0.54* | 0.38* | 0.88* | 0.31* |
| Rhfactor | 50 | 232 | 185 | 47 | 0.85* | 0.63* | 0.59* | 0.23* |
| Pulse (BPM) | 80.80±16.40 | 106.19±18.57 | 105.82±18.72 | 107.7±18.05 | 0.000 | 0.000 | 0.000 | 0.52 |
| SpO2(%) | 98.37±0.67 | 94.26±8.41 | 97.27±0.31 | 81.67±1.43 | 0.000 | 0.001 | 0.000 | 0.000 |
| RBC (×1012/L) | 4.66±0.53 | 4.65±1.10 | 4.29±0.65 | 6.11±1.33 | 0.92 | 0.000 | 0.000 | 0.000 |
| MCV (fl) | 87.77±13.85 | 78.12±10.98 | 77.37±11.53 | 81.1±7.87 | 0.000 | 0.000 | 0.004 | 0.01 |
| RDW (%) | 13.98±2.63 | 14.34±3.61 | 14.13±3.72 | 15.17±2.99 | 0.42 | 0.74 | 0.04 | 0.045 |
| HCT (%) | 40.72±6.78 | 36.87±9.50 | 33.88±5.67 | 49.01±12.02 | 0.001 | 0.000 | 0.000 | 0.000 |
| PLT (×109/L) | 220.52±94.44 | 245.04±95.25 | 254.27±95.58 | 207.5±84.91 | 0.10 | 0.03 | 0.47 | 0.002 |
| HGB (g/dL) | 12.99±2.07 | 11.97±2.71 | 11.11±1.79 | 15.46±3.01 | 0.004 | 0.000 | 0.000 | 0.000 |
| MCH (g/dL) | 28.01±3.77 | 26.25±4.43 | 26.10±2.97 | 26.81±7.97 | 0.0048 | 0.002 | 0.35 | 0.55 |
| MCHC (g/dL) | 32.05±2.03 | 32.87±1.97 | 33.16±1.67 | 31.71±2.59 | 0.01 | 0.001 | 0.48 | 0.001 |
| LYM (× 109/L) | 2.46±0.93 | 9.44±3.13 | 9.45±3.14 | 9.37±3.16 | 0.000 | 0.000 | 0.000 | 0.86 |
| LYM (%) | 36.62±11.96 | 47.79±13.45 | 47.55±13.67 | 47.25±12.67 | 0.000 | 0.000 | 0.000 | 0.88 |
| PLR | 96.69±41.16 | 27.42±11.11 | 28.45±11.29 | 23.24±9.35 | 0.000 | 0.000 | 0.000 | 0.002 |
Data of age are presented as median (range), gender, and blood group are presented as numbers, and rest parameters are presented as mean±standard deviation. *Fisher’s exact test P-value. Significant P-values are denoted in bold font. Acyn: Acyanotic, BMI: Body mass index, BPM: Beats per minute, Cyn: Cyanotic, HCT: Hematocrit, HGB: Hemoglobin, LYM: Lymphocyte, MCV: Mean corpuscular volume, MCH: Mean corpuscular hemoglobin, MCHC: Mean corpuscular hemoglobin concentration, PLT: Platelet, PLR: Platelettolymphocyte ratio, RBC: Red blood cell, RDW: Red cell distribution width, SpO2: Oxygen saturation
Statistical analysis
The data were analyzed using the IBM Statistical Package for the Social Sciences 21.0 and MS Excel. For continuous variables, readings are presented as mean ± standard deviation to denote normally distributed data and median (range) for non-normal distributed datasets. A Student’s t-test was performed for the case–control association of continuous data. A Chi-square test or Fisher’s exact test (if count was <5) was performed for categorical data. A multivariate logistic regression was adopted to compute P values. The strength of the correlation was determined by Chan.[12] A P < 0.05 was considered statistically significant.
RESULTS
Characteristics of the subjects
A total of 238 CHD patients and 50 healthy controls who fulfilled the inclusion criteria were recruited [Table 1]. The most common phenotype was VSD (30.6%), followed by ASD (24.3%), PDA (17.2%), TOF (14.6%), and misc. (13.3%). There were 145 (60.9%) males and 93 (39.1%) females. No significant difference was seen in gender distribution between cases and controls. The most abounded blood group was B (37.8%; P = 0.055). The median age at detection of CHD was 360 days (range, 0–10800 days), the median age at treatment was 484 days (range, 0–16065 days), the median ventilation stay was 3 h (range, 0–4 h), the median ICU stay was 3 days (range, 0–8 days), and median total hospital stay was 7 days (range, 4–24 days). CBC parameters include red blood cell (RBC), mean corpuscular volume (MCV), red cell distribution width (RDW), hematocrit (HCT), PLT, hemoglobin (HGB), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and lymphocytes (LYM).
Hematological profile
Association of clinical and laboratory parameters in case-control
Body mass index (BMI), pulse, oxygen saturation (SpO2), and CBC indices were compared between cases and controls [Table 1]. BMI was significantly lower (13.54 ± 0.17 vs. 21.99 ± 0.69; P = 0.000), and pulse was a bit higher (106.19 ± 18.57 vs. 80.80 ± 16.40; P = 0.000) in all cases. SpO2 was significantly dropped in all cases but a major change was observed in the cyanotic category (P = 0.000). Cyanotic cases showed an increase in RBC, HCT, and HGB, while acyanotic cases reported a decrease in RBC, HCT, and HGB (P = 0.000). PLT and MCHC were increased in acyanotic cases P = 0.03 and P = 0.001, respectively. Both acyanotic and cyanotic groups had shown elevated RDW % (P = 0.74 in acyn; P = 0.04 in cyn), LYM counts (P = 0.000), and LYM % (P = 0.000) and lowered MCV (P = 0.000 in acyn; P = 0.004 in cyn) and MCH (P = 0.002 in acyn; P = 0.35 in cyn). A significant association was observed between acyanotic and cyanotic groups for RBC (P = 0.000), MCV (P = 0.01), RDW % (P = 0.045), HCT (P = 0.000), PLT (P = 0.002), HGB (P = 0.000), and MCHC (P = 0.001). PLR was also studied and found a drastic significant drop in all cases, acyn and cyn cases with P = 0.000, and P = 0.002 for acyn versus cyn category.
Association among CHD sub-phenotypes
Major five groups were made, namely VSD, ASD, TOF, PDA, and misc. LYM counts and LYM % were significantly high (P < 0.05), and MCV was found low (P < 0.05) in all groups [Table 2]. VSD, ASD, and PDA followed a similar pattern with most of the parameters. In all three categories, a significant drop for RBC (P < 0.001), HCT (P = 0.000), HGB (P = 0.000), and MCH (P < 0.01; except for ASD, P = 0.22) and elevated readings for PLT (P = 0.002 only for PDA) and MCHC (P < 0.05) was obtained. In TOF cases, RBC, HCT, and HGB were increased (P = 0.000), while PLT (P = 0.2), MCH (P = 0.006), and MCHC (P = 0.49) were decreased. Other than LYM counts and LYM %, only MCV was significantly decreased (P = 0.004) in the misc category.
| Variable | Controls (n=50) | VSD (n=73) | ASD (n=58) | TOF (n=35) | PDA (n=40) | Miscellaneous (n=32) | P-values (w.r.t. control) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VSD | ASD | TOF | PDA | Misc. | |||||||
| BMI | 21.99±0.69 | 13.02±1.88 | 13.74±2.36 | 14.16±4.21 | 13.78±2.71 | 13.35±1.42 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Pulse (BPM) | 80.80±16.40 | 105.97±18.17 | 96.62±13.39 | 104.54±16.21 | 115.83±17.56 | 113.5±22.74 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| SpO2(%) | 98.37±0.67 | 97.27±3.61 | 98.1±1.41 | 79.88±9.65 | 98.36±0.79 | 90.41±9.79 | 0.01 | 0.21 | 0.000 | 0.98 | 0.000 |
| RBC (×1012/L) | 4.66±0.53 | 4.29±0.54 | 4.27±0.54 | 6.46±1.27 | 4.21±0.49 | 4.73±1.19 | 0.0003 | 0.0003 | 0.000 | 0.000 | 0.76 |
| MCV (fl) | 87.77±13.85 | 76.67±8.61 | 81.66±7.65 | 82±8.17 | 72.21±14.44 | 77.91±14.79 | 0.000 | 0.007 | 0.02 | 0.000 | 0.004 |
| RDW (%) | 13.98±2.63 | 14.37±4.37 | 13.46±3.10 | 15.09±2.89 | 14.32±3.08 | 15.01±3.69 | 0.53 | 0.35 | 0.07 | 0.57 | 0.18 |
| HCT (%) | 40.72±6.78 | 33.57±5.14 | 34.99±4.36 | 51.95±11.74 | 31.35±3.37 | 38.17±9.92 | 0.000 | 0.000 | 0.000 | 0.000 | 0.21 |
| PLT (×109/L) | 220.52±94.44 | 251.39±87.02 | 232.98±85.46 | 198.08±66.79 | 291.09±108.71 | 246.14±113.95 | 0.07 | 0.47 | 0.2 | 0.002 | 0.29 |
| HGB (g/dL) | 12.99±2.07 | 10.98±1.50 | 11.61±1.39 | 16.38±2.64 | 10.13±1.74 | 12.33±2.59 | 0.000 | 0.0001 | 0.000 | 0.000 | 0.23 |
| MCH (g/dL) | 28.01±3.77 | 25.63±2.54 | 27.21±2.74 | 25.94±3.01 | 24.67±3.22 | 28.08±9.37 | 0.0002 | 0.22 | 0.006 | 0.000 | 0.96 |
| MCHC (g/dL) | 32.05±2.03 | 33.35±1.93 | 33.26±1.08 | 31.68±2.64 | 32.95±1.46 | 32.32±2.40 | 0.0005 | 0.0003 | 0.49 | 0.02 | 0.59 |
| LYM (×109/L) | 2.46±0.93 | 9.69±2.81 | 8.95±3.02 | 8.95±2.94 | 9.57±3.34 | 10.12±3.87 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| LYM (%) | 36.62±11.96 | 49.46±13.57 | 41.81±12.72 | 45.94±12.17 | 51.83±12.45 | 49.56±14.14 | 0.000 | 0.03 | 0.001 | 0.000 | 0.000 |
| PLR | 96.69±41.16 | 26.94±8.99 | 27.38±9.99 | 23.52±8.94 | 31.82±10.30 | 27.32±17.56 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Data are presented as mean±standard deviation. Significant P-values are denoted in bold font. ASD: Atrial septal defect, BMI: Body mass index, BPM: Beats per minute, HCT: Hematocrit, HGB: Hemoglobin, LYM: Lymphocyte, Misc: Miscellaneous, MCV: Mean corpuscular volume, MCH: Mean corpuscular hemoglobin, MCHC: Mean corpuscular hemoglobin concentration, PDA: Patent ductus arteriosus, PLT: Platelet, PLR: Platelettolymphocyte ratio, RBC: Red blood cell, RDW: Red cell distribution width, TOF: Tetralogy of Fallot, VSD: Ventricular septal defect, CHD: Congenital heart disease, SpO2: Oxygen saturation
Gender-wise association study
Both genders showed similar significant drops in BMI (P = 0.000) and SpO2 (P = 0.000) and elevated pulse rate (P = 0.000) [Table 3]. MCV (P < 0.001) and HCT (P < 0.05) were decreased, and MCHC (P < 0.05), LYM counts (P = 0.000), and LYM % (P = 0.000) were increased in both sexes. RBC (P = 0.03) and HGB (P = 0.000) were significantly dropped in females, while MCH was significantly lowered in males (P = 0.000). To check any difference in the genders among acyanotic and cyanotic categories, a separate analysis was performed, which highlighted the association in RBC (P = 0.008; higher in males) among acyanotic group and LYM counts (P = 0.047; higher in females) among the cyanotic group.
| Variable | Control (n=50) | Cases | Acyanotic | Cyanotic | P-values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (n=145) | Female (n=93) | Males | Females | Males | Females | Male vs. control | Female vs. control | Male vs. Female | Acyn (Male vs. Female) | Cyn (Male vs. Female) | ||
| BMI | 21.99±0.69 | 13.67±2.77 | 13.34±2.22 | 13.45±2.13 | 13.41±2.36 | 14.35±4.18 | 12.94±1.21 | 0.000 | 0.000 | 0.31 | 0.90 | 0.07 |
| Pulse (BPM) | 80.80±16.40 | 105.71±19.05 | 106.9±17.77 | 104.65±19.09 | 107.24±17.88 | 108.03±18.30 | 106.62±17.27 | 0.000 | 0.000 | 0.62 | 0.34 | 0.81 |
| SpO2(%) | 98.37±0.67 | 93.09±9.58 | 95.84±6.25 | 97.05±4.89 | 80.54±10.06 | 97.53±3.47 | 85.31±9.04 | 0.000 | 0.0002 | 0.008 | 0.43 | 0.13 |
| RBC (×1012/L) | 4.66±0.53 | 4.81±1.16 | 4.39±0.94 | 4.39±0.73 | 4.15±0.46 | 6.13±1.24 | 5.89±1.67 | 0.23 | 0.03 | 0.003 | 0.008 | 0.64 |
| MCV (fl) | 87.77±13.85 | 77.24±11.52 | 79.44±9.95 | 76.68±10.88 | 79.17±10.45 | 80.64±8.51 | 81.63±6.22 | 0.000 | 0.0003 | 0.12 | 0.12 | 0.66 |
| RDW (%) | 13.98±2.63 | 14.52±3.72 | 14.04±3.40 | 14.35±4.01 | 13.81±3.33 | 15.11±2.67 | 15.39±3.77 | 0.27 | 0.91 | 0.31 | 0.32 | 0.81 |
| HCT (%) | 40.72±6.78 | 37.81±10.12 | 35.29±8.26 | 34.33±6.64 | 33.22±3.86 | 49.07±11.01 | 47.48±15.32 | 0.02 | 0.000 | 0.04 | 0.15 | 0.74 |
| PLT (×109/L) | 220.52±94.44 | 245.89±97.03 | 244.55±92.73 | 256.46±96.26 | 252.90±94.83 | 209.27±91.06 | 202±64.11 | 0.11 | 0.15 | 0.91 | 0.80 | 0.76 |
| HGB (g/dL) | 12.99±2.07 | 12.26±2.97 | 11.49±2.16 | 11.25±2.12 | 10.91±1.67 | 15.5±2.96 | 14.97±3.42 | 0.06 | 0.000 | 0.02 | 0.15 | 0.63 |
| MCH (g/dL) | 28.01±3.77 | 25.81±2.83 | 26.94±6.08 | 25.90±2.73 | 26.45±3.26 | 25.47±3.19 | 30.25±14.04 | 0.0003 | 0.19 | 0.09 | 0.23 | 0.25 |
| MCHC (g/dL) | 32.05±2.03 | 32.82±2.18 | 32.98±1.62 | 33.21±1.93 | 33.13±1.27 | 31.57±2.48 | 32.27±2.93 | 0.03 | 0.006 | 0.51 | 0.73 | 0.46 |
| LYM (×109/L) | 2.46±0.93 | 9.23±3.02 | 9.80±3.29 | 9.47±3.12 | 9.58±3.17 | 8.64±2.57 | 11.14±3.89 | 0.000 | 0.000 | 0.18 | 0.81 | 0.047 |
| LYM (%) | 36.62±11.96 | 47.98±13.56 | 46.73±13.24 | 47.64±14.21 | 47.23±12.74 | 48.37±10.87 | 44.62±16.48 | 0.000 | 0.000 | 0.48 | 0.84 | 0.46 |
| PLR | 96.69±41.16 | 27.99±11.56 | 26.49±10.29 | 28.47±10.42 | 27.75±10.24 | 24.68±9.60 | 19.84±7.67 | 0.000 | 0.000 | 0.29 | 0.64 | 0.08 |
Data are presented as mean±standard deviation. Significant P-values are denoted in bold font. Acyn: Acyanotic, BMI: Body mass index, BPM: Beats per minute, Cyn: Cyanotic, HCT: Hematocrit, HGB: Hemoglobin, LYM: Lymphocyte, MCV: Mean corpuscular volume, MCH: Mean corpuscular hemoglobin, MCHC: Mean corpuscular hemoglobin concentration, PLT: Platelet, PLR: Platelettolymphocyte ratio, RBC: Red blood cell, RDW: Red cell distribution width, SpO2: Oxygen saturation
Multivariate analysis using logistic regression
The study revealed that the strongest positive correlations were obtained between HGB and HCT (r = 0.92), followed by RBC with HCT (r = 0.88) and HGB (r = 0.83). SpO2 was found to be strongly negatively correlated with RBC (r = −0.78), HCT (r = −0.76), and HGB (r = −0.73) [Figure 1]. The correlation of CBC parameters with age at diagnosis of CHDa, age at interventionb, ventilation time-periodc, ICU stayd, total hospital stay of patiente, and overall category (including all a to e) was also investigated within all cases and acyanotic and cyanotic sub-categories [Table 4]. Strong correlations were observed in all three classes for pulse rate (r = 0.61, 0.63, and 0.65, respectively) and LYM % (r = 0.52, 0.54, and 0.62, respectively) in the overall category. In the same category, a moderate correlation was observed for BMI (r = 0.29), SpO2 (r = 0.40), RBC (r = 0.27), MCV (r = 0.39), HCT (r = 0.38), PLT (r = 0.35), HGB (r = 0.41), MCH (r = 0.27), MCHC (r = 0.24), and LYM (r = 0.39) in all cases group. BMI, SpO2, and MCV were positively correlated with age at diagnosis (P = 0.003, P = 0.01, and P = 0.000, respectively) and age at intervention (P = 0.001, P = 0.03, and P = 0.000, respectively) while pulse rate, PLT, LYM, and LYM % were negatively correlated with age at diagnosis (P = 0.000) and age at intervention (P = 0.000) in all cases. Furthermore, HGB (P = 0.01) and MCH (P = 0.006) were found positively correlated with age at diagnosis, and RBC (P = 0.01) was found inversely related to age at intervention. In acyanotic group, BMI, MCV, HCT, HGB, and MCH were positively, and pulse rate, PLT, LYM, and LYM % were inversely related to the age at detection and intervention. RBC showed the same inverse relation with age at intervention (P = 0.008). MCV was positively, and pulse rate, RDW %, and LYM % were negatively associated with diagnosis and intervention age in cyanotic class. MCHC was found positively correlated (except for the cyanotic category), and HCT was negatively correlated (only in acyanotic category) with ventilation stay. RBC (P = 0.001), MCV (P = 0.05), HCT (P = 0.000), and HGB (P = 0.000) were positively, and SpO2 (P = 0.000) and PLR (P = 0.04) were negatively related to the patient’s ICU stay.
| Variable | Overall (a, b, c, d and e) r (P-value) | Age at diagnosis (a) Type of correlation (P-value) | Age at intervention (b) Type of correlation (P-value) | Ventilation stay (c) Type of correlation (P-value) | ICU stay (d) Type of correlation (P-value) | Total stay at hospital (e) Type of correlation (P-value) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All cases | Acyn | Cyn | All cases | Acyn | Cyn | All cases | Acyn | Cyn | All cases | Acyn | Cyn | All cases | Acyn | Cyn | All cases | Acyn | Cyn | |
| BMI | 0.29 (0.003) | 0.39 (0.000) | 0.32 (0.59) | +ve (0.003) | +ve (0.000) | −ve (0.19) | +ve (0.0006) | +ve (0.0002) | +ve (0.32) | −ve (0.69) | +ve (0.97) | −ve (0.51) | +ve (0.76) | −ve (0.69) | +ve (0.99) | +ve (0.55) | +ve (0.23) | −ve (0.69) |
| Pulse (BPM) | 0.61 (0.000) | 0.63 (0.000) | 0.65 (0.0006) | −ve (0.000) | −ve (0.000) | −ve (0.000) | −ve (0.000) | −ve (0.000) | −ve (0.002) | +ve (0.08) | +ve (0.07) | +ve (0.80) | −ve (0.50) | −ve (0.59) | −ve (0.94) | +ve (0.29) | +ve (0.73) | +ve (0.13) |
| SpO2(%) | 0.40 (0.000) | 0.19 (0.33) | 0.30 (0.68) | +ve (0.01) | +ve (0.06) | +ve (0.46) | +ve (0.03) | +ve (0.07) | +ve (0.70) | −ve (0.18) | −ve (0.98) | −ve (0.22) | −ve (0.000) | −ve (0.36) | −ve (0.26) | +ve 0.59) | +ve (0.31) | +ve (0.72) |
| RBC (×1012/L) | 0.27 (0.007) | 0.29 (0.01) | 0.30 (0.66) | −ve (0.91) | +ve (0.07) | −ve (0.35) | −ve (0.01) | −ve (0.008) | −ve (0.83) | +ve (0.88) | −ve (0.27) | +ve (0.47) | +ve (0.001) | +ve (0.62) | +ve (0.41) | −ve 0.28) | −ve (0.93) | −ve (0.11) |
| MCV (fl) | 0.39 (0.000) | 0.39 (0.000) | 0.63 (0.001) | +ve (0.000) | +ve (0.000) | +ve (0.000) | +ve (0.0004) | +ve (0.001) | +ve (0.001) | +ve (0.38) | +ve (0.41) | +ve (0.49) | +ve (0.05) | +ve (0.39) | +ve (0.06) | +ve 0.68) | +ve (0.37) | −ve (0.23) |
| RDW (%) | 0.16 (0.45) | 0.19 (0.37) | 0.57 (0.01) | −ve (0.32) | −ve (0.89) | −ve (0.001) | −ve (0.06) | −ve (0.36) | −ve (0.002) | −ve (0.18) | −ve (0.29) | −ve (0.29) | +ve (0.87) | −ve (0.21) | +ve (0.21) | −ve (0.74) | +ve (0.91) | −ve (0.34) |
| HCT (%) | 0.38 (0.000) | 0.44 (0.000) | 0.28 (0.76) | +ve (0.14) | +ve (0.000) | +ve (0.63) | +ve (0.33) | +ve (0.006) | +ve (0.29) | −ve (0.30) | −ve (0.007) | +ve (0.98) | +ve (0.000) | +ve (0.04) | +ve (0.14) | −ve (0.18) | −ve (0.33) | −ve (0.18) |
| PLT (×109/L) | 0.35 (0.000) | 0.38 (0.000) | 0.37 (0.39) | −ve (0.0004) | −ve (0.000) | +ve (0.97) | −ve (0.0004) | −ve (0.0004) | −ve (0.15) | +ve (0.69) | +ve (0.41) | −ve (0.58) | −ve (0.03) | −ve (0.65) | −ve (0.059) | +ve (0.79) | −ve (0.75) | +ve (0.15) |
| HGB (g/dL) | 0.41 (0.000) | 0.43 (0.000) | 0.41 (0.25) | +ve (0.01) | +ve (0.000) | +ve (0.18) | +ve (0.83) | +ve (0.007) | +ve (0.09) | +ve (0.35) | −ve (0.42) | +ve (0.11) | +ve (0.000) | +ve (0.09) | +ve (0.11) | −ve (0.64) | +ve (0.99) | −ve (0.23) |
| MCH (g/dL) | 0.27 (0.006) | 0.33 (0.002) | 0.22 (0.91) | +ve (0.006) | +ve (0.0005) | +ve (0.69) | +ve (0.09) | +ve (0.01) | −ve (0.92) | +ve (0.38) | +ve (0.12) | −ve (0.93) | +ve (0.054) | +ve (0.39) | +ve (0.27) | +ve (0.89) | +ve (0.42) | −ve (0.83) |
| MCHC (g/dL) | 0.24 (0.03) | 0.27 (0.04) | 0.39 (0.30) | +ve (0.17) | +ve (0.26) | +ve (0.21) | +ve (0.25) | +ve (0.42) | +ve (0.14) | +ve (0.0004) | +ve (0.0005) | +ve (0.16) | −ve (0.26) | −ve (0.64) | −ve (0.13) | +ve (0.15) | +ve (0.16) | +ve (0.31) |
| LYM (×109/L) | 0.39 (0.000) | 0.45 (0.000) | 0.42 (0.23) | −ve (0.000) | −ve (0.000) | +ve (0.75) | −ve (0.000) | −ve (0.000) | −ve (0.12) | +ve (0.97) | +ve (0.83) | −ve (0.56) | +ve (0.96) | +ve (0.17) | −ve (0.19) | +ve (0.89) | −ve (0.19) | +ve (0.02) |
| LYM (%) | 0.52 (0.000) | 0.54 (0.000) | 0.62 (0.002) | −ve (0.000) | −ve (0.000) | −ve (0.0001) | −ve (0.000) | −ve (0.000) | −ve (0.008) | +ve (0.99) | −ve (0.57) | +ve (0.25) | −ve (0.24) | −ve (0.12) | +ve (0.55) | −ve (0.57) | +ve (0.96) | −ve (0.11) |
| PLR | 0.17 (0.35) | 0.09 (0.96) | 0.24 (0.86) | +ve (0.82) | +ve (0.95) | −ve (0.72) | +ve (0.89) | −ve (0.87) | +ve (0.87) | −ve (0.99) | −ve (0.99) | +ve (0.81) | −ve (0.04) | −ve (0.27) | −ve (0.34) | +ve (0.65) | +ve (0.55) | −ve (0.79) |
Values are represented as Pearson correlation coefficient (P-value). (+) sign denotes positive correlation and (−) sign denotes negative correlation. Significant P-values are denoted in bold font. Acyn: Acyanotic, BMI: Body mass index, BPM: Beats per minute, Cyn: Cyanotic, HCT: Hematocrit, HGB: Hemoglobin, LYM: Lymphocyte, MCV: Mean corpuscular volume, MCH: Mean corpuscular hemoglobin, MCHC: Mean corpuscular hemoglobin concentration, PLT: Platelet, PLR: Platelettolymphocyte ratio, RBC: Red blood cell, RDW: Red cell distribution width, SpO2: Oxygen saturation, CBC: Complete blood count, ICU: Intensivecare unit
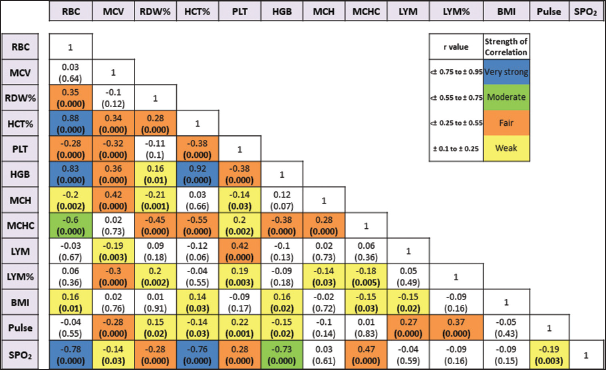
- Multivariate logistic regression of hematological indices of congenital heart disease cases. BMI: Body mass index, HCT: Hematocrit, HGB: Hemoglobin, LYM: Lymphocyte, MCV: Mean corpuscular volume, MCH: Mean corpuscular hemoglobin, MCHC: Mean corpuscular hemoglobin concentration, PLT: Platelet, RBC: Red blood cell, RDW: Red cell distribution width, SpO2: Oxygen saturation
DISCUSSION
This study highlighted the association of CBC indices and related factors with CHD from the North Indian region [Figure 2]. The effect of iron intake on hematological parameters in cyanotic CHD patients is one of the studies from India.[13] Inflammatory actions are significantly associated with the pathophysiology of cardiac arrest, cardiomyocyte dysfunction, apoptosis, and fibrosis, therefore, it could serve as an innovative therapeutic target for cardiovascular defects.[14] Previous studies suggested lymphocytosis association with cardiac failure among adults which also showed the same pattern with pediatric CHD population in the current study (P = 0.000), therefore can be an ideal biomarker to predict any CHD subphenotype.[15] PLT can be an independent prognostic predictor to assess disease prognosis for acyanotic CHD cases, particularly PDA (P = 0.002), and showed significant deviation from the cyanotic CHD population (P = 0.002). Previous studies indicated that cyanotic patients often experience low PLT counts and are prone to thrombocytopenia. Our study corroborated these findings; however, the decrease was not significant (P = 0.47).[16] PLR has also been used to predict heart failure, cardiac anomalies, and renal disorders which were found to significantly drop (P = 0.000) irrespective of categories (acyn or cyn), sub-phenotypes, and gender.[17-19] Anemic CHD patients are prone to have a high mortality rate, and iron deficiency is common in cyanotic CHD, but our cyanotic patients showed elevated RBC, HGB, HCT, and RDW, which might be due to distinct populations.[20] Other RBC-related components MCV (P = 0.004), MCH (P = 0.35), and MCHC (P = 0.48) followed a reverse relationship, i.e., decline in the cyanotic group. MCV and RDW were decreased (P = 0.000) and increased (P = 0.42) in all cases, respectively, and both can act as clinical markers for predicting the occurrence and prognosis of cardiovascular disease.[21] A recent study on acyanotic CHD patients from Tehran also found lower HGB, but the result was statistically insignificant (P = 0.83).[22] Exact anemic status can be determined by considering other parameters such as serum ferritin, total iron-binding capacity, and transferrin levels.[23] The higher BMI of the patients and elevated values of SpO2, MCV, HGB, and MCH may contribute to late diagnosis and intervention of CHD, while higher pulse rate, PLT counts, LYM, and LYM % may help in early diagnosis. Furthermore, higher MCHC can cause longer ventilation time, while higher HCT reduces the chance of ventilation. The increased level of RBC, MCV, HCT, and HGB and decreased level of SpO2 and PLR can result in longer ICU stays after intervention [Figure 3]. Other studies have reported that neutropenia, lymphopenia, thrombocytopenia, and NLR may result in longer hospital stays and longer ventilation periods increased ICU stays.[7,15,17] The higher pre-operative RDW % may cause adverse effects after intervention and can even result in heart failure.[24]
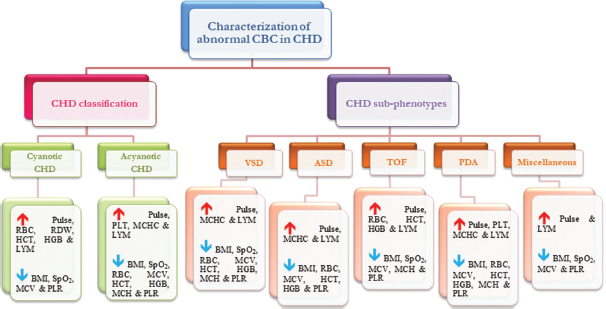
- An overview of hematological profile of congenital heart disease patients. CBC: Complete blood count, ASD: Atrial septal defect, BMI: Body mass index, BPM: Beats per minute, HCT: Hematocrit, HGB: Hemoglobin, LYM: Lymphocyte, Misc: Miscellaneous, MCV: Mean corpuscular volume, MCH: Mean corpuscular hemoglobin, MCHC: Mean corpuscular hemoglobin concentration, PDA: Patent ductus arteriosus, PLT: Platelet, PLR: Platelet-to-lymphocyte ratio, RBC: Red blood cell, RDW: Red cell distribution width, TOF: Tetralogy of Fallot, VSD: Ventricular septal defect, CHD: Congenital heart disease, SpO2: Oxygen saturation
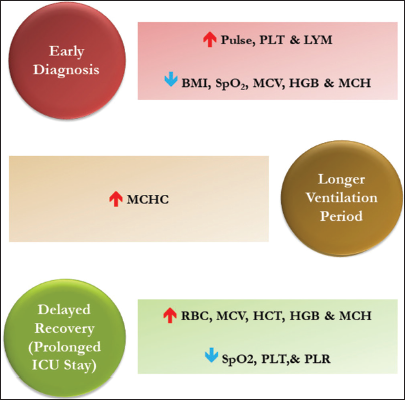
- Impact of abnormal complete blood count indices on diagnosis and hospitalization. BMI: Body mass index, HCT: Hematocrit, HGB: Hemoglobin, LYM: Lymphocyte, MCV: Mean corpuscular volume, MCH: Mean corpuscular hemoglobin, PLT: Platelet, PLR: Platelet-to-lymphocyte ratio, RBC: Red blood cell, SpO2: Oxygen saturation, MCHC: Mean corpuscular hemoglobin concentration.
The death rates from CHD rose as the sociodemographic index decreased. India, China, Pakistan, and Nigeria, accounting for 39.7% of global mortality, exhibited the highest mortality rates, and the average rate of cardiac surgery is merely 0.5 per million people.[25,26] The primary factors hindering progress are thought to be inadequate access to congenital cardiac care and malnutrition. Anderson et al. proposed a predictor model of cardiovascular disease using CBC-derived risk score, so the next approach is to prepare a personalized model for CHD.[27] Sustainable Development Goals 3.2 and 3.4 target reducing the mortality of newborns to <12/1000 and children to <25/1000 live births and minimize premature mortality from non-communicable diseases by one-third by 2030.[28] Since CHD represents nearly one-third of all congenital defects, therefore, the focus on CHD is integral to eliminating preventable child deaths.[29,30] In India, every year, over 0.2 million babies are born with CHD, and among them, nearly 20% require cardiac interventions within the first year of birth; hence, early detection plays a vital role in the overall survival rate. The cheaper and wide availability of the CBC test shows its advantage over expensive and sensitive tests for disease diagnosis in such countries.
CONCLUSION
Our findings will help in predicting adverse conditions of patients for timely diagnosis, reduction of post-surgery mortality, and prevention of CHD. Furthermore, the scope to explore other complex inflammatory indices, hematological and biochemical parameters such as C-reactive protein, eosinophil sedimentation ratio, fasting blood sugar neutrophil counts, NLR, MLR, mean platelet volume, urea, creatinine, serum glutamine transaminase, and serum glutamic-oxaloacetic transaminase can give more insight into understanding the whole system to design a predictive model for the overall outcome of the abnormal blood and biochemical parameters and ultimately to prevent the mortality and morbidity of the patient with CHD. Although the number of controls was adequate for CHD sub-phenotypes comparison, the scarcity of age-matched healthy controls when compared with all CHD cases was another limiting factor of this study. The study findings’ reproducibility at a larger sample size could give a more validated conclusion.
Acknowledgments
The authors acknowledge the contribution of the Cardiology and Surgery teams and laboratory technicians of the institution for sharing the clinical findings and performing CBC, respectively.
Author contributions
S.A. and P.K. conceptualization; S.A. designing of the study; A.T., A.S., and P.S. provided the study samples; A.K. data acquisition; S.A. data curation, statistical analysis, and manuscript preparation; P.K. reviewing. All authors read and approved the final version of the manuscript. P.K. and S.A. take the overall charge of the article.
Ethical approval
The research/study was approved by the Institutional Review Board at Sri Sathya Sai Sanjeevani Research Foundation, number PS00002/IEC/5/2018, dated May 13, 2018.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)- assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Management of Birth Defects and Haemoglobin Disorders: Report of a Joint WHO-March of Dimes Meeting. 2006. Available from: http://www.who.int/genomics/publications/WHO-MODreportfinal.pdf?ua=1 [Last accessed on 2024 Jan 10]
- [Google Scholar]
- Congenital Heart Disease in India: A Status Report. Indian Pediatr. 2018;55:1075-82.
- [CrossRef] [Google Scholar]
- Clinical Profile of Congenital Heart Diseases Detected in a Tertiary Hospital in China: A Retrospective Analysis. Front Cardiovasc Med. 2023;10:1131383.
- [CrossRef] [Google Scholar]
- March of Dimes Global Report on Birth Defects: The Hidden Toll of Dying and Disabled Children. New York: The March of Dimes Birth Defects Foundation; 2006. Available from: http://www.marchofdimes.org/materials/global-report-on-birth-defects-the-hidden-toll-of-dying-and-disabled-children-executive-summary.pdf [Last accessed on 2024 Jan 07]
- [Google Scholar]
- The Incidence of Congenital Heart Disease. J Am Coll Cardiol. 2002;39:1890-900.
- [CrossRef] [Google Scholar]
- Genetic Basis for Congenital Heart Disease: Revisited: A Scientific Statement From the American Heart Association. Circulation. 2018;138:e653-711.
- [CrossRef] [Google Scholar]
- The Beneficial Role of Simple Inflammatory Blood Indices in Pediatric Cardiology. Adv Clin Exp Med. 2023;32:1041-8.
- [CrossRef] [Google Scholar]
- The Neutrophil-to-lymphocyte Ratio and Mean Platelet Volume Can Be Associated with Severity of Valvular Involvement in Patients with Acute Rheumatic Carditis. Cardiovasc J Afr. 2018;29:296-300.
- [CrossRef] [Google Scholar]
- Do Platelet-to-lymphocyte Ratio (PLR) and Neutrophil-to-lymphocyte Ratio (NLR) Have a Predictive Value on Pediatric Extracorporeal Membrane Oxygenation (ECMO) Results? Cardiol Young. 2021;31:1003-8.
- [CrossRef] [Google Scholar]
- Haematological Considerations in Patients with Cyanotic Congenital Heart Disease: A Review. Dent Update. 2006;33(617-8):620->2.
- [CrossRef] [Google Scholar]
- Hematological Parameters as Prognostic Biomarkers in Patients with Cardiovascular Diseases. Curr Cardiol Rev. 2019;15:274-82.
- [CrossRef] [Google Scholar]
- Cyanotic Congenital Heart Disease Effects of Iron Supplementation on Hematological Indices in Iron-sufficient and Iron-deficient Children. J Indian Coll Cardiol. 2021;11:193-7.
- [CrossRef] [Google Scholar]
- Exaggerated Inflammation and Monocytosis Associate with Diastolic Dysfunction in Heart Failure with Preserved Ejection Fraction: Evidence of M2 Macrophage Activation in Disease Pathogenesis. J Card Fail. 2015;21:167-77.
- [CrossRef] [Google Scholar]
- Platelet, Neutrophil and Lymphocyte Quantitative Abnormalities in Patients with Heart Failure: A Retrospective Study. Vasc Health Risk Manag. 2023;19:69-78.
- [CrossRef] [Google Scholar]
- Pathogenesis of Thrombocytopenia in Cyanotic Congenital Heart Disease. Am J Cardiol. 2006;98:254-8.
- [CrossRef] [Google Scholar]
- The Impact of Platelet-tolymphocyte Ratio on Clinical Outcomes in Heart Failure: A Systematic Review and Meta-analysis. Ther Adv Cardiovasc Dis. 2024;18:17539447241227287.
- [CrossRef] [Google Scholar]
- The Predictive Value of NLR, MLR, and PLR in the Outcome of End-Stage Kidney Disease Patients. Biomedicines. 2022;10:1272.
- [CrossRef] [Google Scholar]
- Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio as Useful Predictive Markers of Prediabetes and Diabetes Mellitus. Diabetes Metab Syndr. 2017;11(Suppl 1):S127-31.
- [CrossRef] [Google Scholar]
- Diagnosis and Management of Noncardiac Complications in Adults With Congenital Heart Disease: A Scientific Statement from the American Heart Association. Circulation. 2017;136:e348-92.
- [CrossRef] [Google Scholar]
- Usefulness of Complete Blood Count (CBC) to Assess Cardiovascular and Metabolic Diseases in Clinical Settings: A Comprehensive Literature Review. Biomedicines. 2022;10:2697.
- [CrossRef] [Google Scholar]
- Hematological Indices in Pediatric Patients with Acyanotic Congenital Heart Disease: A Cross-sectional Study of 248 Patients. Egypt J Med Hum Genet. 2022;23:47.
- [CrossRef] [Google Scholar]
- Iron Deficiency Anemia In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448065 [Last accessed on 2024 Jul 01]
- [Google Scholar]
- Red Blood Cell Distribution Width in Heart Failure: Pathophysiology, Prognostic Role, Controversies and Dilemmas. J Clin Med. 2022;11:1951.
- [CrossRef] [Google Scholar]
- Global, Regional, and National Time Trends in Mortality for Congenital Heart Disease, 1990-2019: An Age-period-cohort Analysis for the Global Burden of Disease 2019 study. EClinicalMedicine. 2022;43:101249.
- [CrossRef] [Google Scholar]
- Usefulness of a Complete Blood Count-derived Risk Score to Predict Incident Mortality in Patients with Suspected Cardiovascular Disease. Am J Cardiol. 2007;99:169-74.
- [CrossRef] [Google Scholar]
- Global Indicator Framework for the Sustainable Development Goals and targets of the 2030 Agenda for Sustainable Development. 2017. New York: United Nations; Available from: https://unstats.un.org/sdgs/indicators/global%20indicator%20framework%20after%202020%20review_eng.pdf [Last accessed on 2024 Jan 08]
- [Google Scholar]
- NCD Countdown 2030: Worldwide Trends in Non-communicable Disease Mortality and Progress towards Sustainable Development Goal Target 3.4. Lancet. 2018;392:1072-88.
- [CrossRef] [Google Scholar]
- The Invisible Child: Childhood Heart Disease in Global Health. Lancet. 2017;389:16-8.
- [CrossRef] [Google Scholar]
SUPPLEMENTARY TABLE
| CHD sub-phenotype | Count |
|---|---|
| PS | 8 |
| TAPVC | 13 |
| Ventricular septal defect + PS | 4 |
| Aortic stenosis | 2 |
| Transposition of great arteries | 1 |
| Tricuspid atresia | 1 |
| Coarctation of aorta | 1 |
| Sub aortic membrane | 1 |
| Atrioventricular septal defect | 1 |
CHD: Congenital heart disease, PS: Pulmonary stenosis, TAPVC: Total anomalous pulmonary venous connection






