Translate this page into:
Prognostic Cardiac Biomarkers and Tetralogy of Fallot Score: Do they Predict Outcomes in Intracardiac Tetralogy of Fallot Repair?
*Corresponding author: Poonam Malhotra Kapoor, Department of Cardiac Anaesthesia and Critical Care, All India Institute of Medical Sciences, New Delhi, India. docpoonamaiims@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kapoor PM, Singh R, Badge M, Prakash M, Choudhury M, Mujahid OM, et al. Prognostic Cardiac Biomarkers and Tetralogy of Fallot Score: Do they Predict Outcomes in Intracardiac Tetralogy of Fallot Repair? J Card Crit Care TSS. 2024;8:147-54. doi: 10.25259/JCCC_59_2023
Abstract
Objectives:
One of the most common cyanotic congenital heart diseases seen in India is the tetralogy of Fallot (TOF). The presence of chronic hypoxia leads to increased susceptibility to ischemia and infections. The postoperative morbidity and mortality can be predicted earlier, by incorporating various biochemical markers in pre-operative workup, which can minimize post-operative mechanical ventilation and intensive care unit (ICU) stay. We aimed to study 11 different cardiac biomarkers and calculate the All India Institute of Medical Sciences (AIIMS) score as a prognostic marker in TOF patients.
Material and Methods:
After obtaining Institute Ethics Committee approval from the Hospital Ethics Committee with Indian Council of Medical Research (ICMR) Trial No: 5/4/1-1/08-NCD-II and written informed consent, a prospective and observational study was conducted on 150 patients with TOF undergoing elective intra cardiac repair (ICR) divided into two groups. Anesthetic and surgical management was standardized for all patients as per institutional protocol. The data were analyzed in STATA software. The sample size was calculated on the basis of the area under the curve for various biomarkers shown in the previous literature reviews.
Results:
There was a positive correlation between the Endothelin levels 48 h after bypass and post-operative outcome measures such as the duration of inotropes, duration of ventilation, and duration of ICU stay. Pre-cardiopulmonary bypass serum tumor necrosis factor-alpha (TNF-α) showed a significant correlation with mortality in group I patients (P = 0.009) and group II patients (P < 0.05). Intragroup comparison in survivors showed significant changes with time in lactate trends. The mean initial post-operative lactate was significantly lower for survivors than for non-survivors. In addition, the serial mean lactate decreased progressively in all surviving patients compared with non-survivors diagnostic receiver operating characteristic curve for the pressure of oxygen.
Conclusion:
The four biomarkers, namely, Endothelin, TNF-α, BNP, and base excess, were found to be highly sensitive and specific. Using these biomarkers, a score of 2.73 (the AIIMS TOF score) is considered morbid in patients post-ICR in the ICU. The chances of mortality are high, with a sensitivity of 96.9% and specificity of 89.2%.
Keywords
Base excess
Tetralogy of Fallot
Endothelin
Brain natriuretic peptide
Prognostic markers
Mortality
INTRODUCTION
Tetralogy of Fallot (TOF) is an endemic disease in India. Due to the presence of chronic hypoxia, they are most susceptible to ischemia and infections. With intracardiac repair done for its correction on cardiopulmonary bypass (CPB), systemic inflammatory response syndrome (SIRS) sets in, affecting its hemostatic, hepatic, pulmonary, and renal functions, thus worsening heart failure.[1] The majority of the time, clinical features and blood gas analysis reports are used as prognostic markers.[2,3] However, postoperative morbidity and mortality can be predicted earlier by incorporating various biochemical markers in the pre-operative workup, which may help as baseline markers and predict worsening intraoperative and postoperative outcomes of prolonged intensive care unit (ICU) stay and high inotrope use.[4]
Arterial blood gas parameters such as lactate, partial pressure of oxygen (PO2), base excess, and biomarkers such as Endothelin, B natriuretic peptide (BNP), and tumor necrosis factor-alpha (TNF-α), during CPB as prognostic markers for major morbidity in TOF undergoing ICR were evaluated in this study. Measuring their levels was the primary objective, and secondary objectives were determining the total ICU length of stay, the incidence of fibrillation on post-CPB, the duration of ventilator use, duration of inotrope, presence of oliguria, and mortality in the ICU.
Inflammatory markers such as Endothelin and TNF-α are proinflammatory cytokines that have been shown to play a major role in SIRS secondary to CPB.[1-4] It may decrease post CPB according to some in the literature review.[5-10]
Galasko et al.[10] also have suggested that studies on BNP in patients with congenital heart disease should be compared with age and gender-specific normal values.
Objective
Primary objective
Measurement of arterial blood gas parameters – lactate, PO2, and base excess.
Biochemical markers
Endothelin, TNF-α, and brain natriuretic peptide (BNP) levels.
Secondary objectives
Determination of length of ICU stay, the incidence of fibrillation post CPB, duration of postoperative ventilation, inotrope use, oliguria, and mortality.
With the hypothesis that an increase in levels of BNP, Endothelin, PO2, lactate, base excess, and TNF-α preoperatively can also act as a prognostic marker of mortality in patients of TOF. We proceeded to undertake, this study with patients divided into two age-based groups.
MATERIAL AND METHODS
After the Ethics Committee approval (Trial No-5/4/1-1/08-NCD-II), this ICMR-funded project was carried out on 150 patients in the age groups from 1 month to 20 years of age of TOF patients undergoing an ICR. Patients were divided into two groups using a sealed envelope technique. Group I included patients <15 years of age, and Group II which included patients >15 years of age. Patients with preexisting heart failure, clotting disorders, renal dysfunction (serum creatinine than 2 mg/dL) with oliguria, hepatic or nervous system, and immune compromised patients were excluded from the study.
All routine anesthetic and surgical steps were followed as per institutional protocol for all patients. Patients were pre-medicated with oral midazolam 0.5 mg/kg. Patients were induced with injection ketamine (1–2 mg/kg), injection fentanyl (2–3 mcg/kg), and injection rocuronium (0.8–1 mg/kg) to facilitate tracheal intubation. Anesthesia was maintained with sevoflurane (0.5–2%) in an oxygen-air mixture with intermittent doses of fentanyl, midazolam, and cisatracurium infusion. Monitoring included electrocardiogram, pulse oximetry, invasive blood pressure, central venous pressure (CVP), and urine output. Baseline activated clotting time (ACT) was noted before systemic heparinization with 300–400 units/kg of unfractionated heparin to achieve a target ACT of more than 480 s. After surgery, when ACT was near normal and post sternal closure, the patient was shifted to the ICU. Fast-tracking was done in 6 hours as per institutional protocol.
For each patient, age, height, and weight were documented. As per our standard protocol, routine basal heart rate, invasive blood pressure, and CVP were recorded. Arterial blood gas and all biomarkers levels were done at three different time intervals, first after induction (T1), second after protamine administration (T2), and third following 48 h after surgery in the ICU. Hemodynamic variables recorded in the ICU constitute the primary parameters, whereas the secondary outcome measures included duration of post-operative ventilation, the requirement of inotropes and ICU length of stay, urine output, and the presence of any arrhythmias.
Statistical analysis
The sample size was calculated on the basis of area under the curve (AUC) for various biomarkers shown in the previous literature reviews, using the STATA software. The quantitative data were compared by applying a student t-test. If data were not normal, log transformation was applied wherever applicable.
Assuming that AUC is 50% in the patient population for these biomarkers, for example, it is 64% for the bicarbonate (HCO3) biomarker, then from the literature review and if the alpha error is taken as 5% and power of 90, then according to STATA software, 146 cases needs to be enrolled: 73 in the first group and 73 in the second group. In all other parameters with these constraints (type I errors as 5%, power taken as 80%), the sample ranges from 8 to 32. So the maximum of 146 cases rounded, 150 cases considering dropouts were taken.
The data were collected over 10 years, June 2013–May 2023, in a prospective and double-blind manner using the sealed envelope technique.
RESULTS
A total of 150 patients of TOF undergoing ICR were included in the study. Demographic data and perioperative parameters of the participants in both groups (75 each) add similar baseline characteristics [Table 1]. However, in Group I (age <15 years), there was a prolongation of the duration of mechanical ventilation, inotrope use, ICU stay, and hospital stay (P < 0.001) when compared with Group II (age more than 15 years). There were 11 in-hospital deaths (overall mortality 7.3%). Although mortality was higher in Group II, it was not statistically significant (P > 0.05) [Table 1]. The results of each individual biomarker are observed as follows:
| Parameter | Group I n=75 median (range) | Group II n=75 median (range) | P-value |
|---|---|---|---|
| Age (years) | 4 (0.11–14) | 31 (16–55) | - |
| Weight (kg) | 12 (2–56) | 48 (30–85) | - |
| Gender (%) | Females 44 | Females 36 | NS |
| Males 56 | Males 64 | ||
| CPB time (minutes) | 90 (76–104) | 87 (73–104) | NS |
| Clamp time (minutes) | 41 (29–75) | 38 (31–78) | NS |
| Mechanical ventilation (hours) | 17 (8–20) | 10 (6–20) | P<0.001 |
| Inotrope use (days) | 4 (1–5) | 2 (1–5) | P<0.001 |
| ICU stay (days) | 4 (2–6) | 3 (2–7) | P<0.001 |
| Hospital stay (days) | 8 (4–11) | 6 (4–10) | P<0.001 |
| Mortality (%) | 2.7 (n=3) | 5.3 (n=8) | NS |
CPB: Cardiopulmonary bypass, ICU: Intensive care unit
Brain natriuretic peptide (BNP)
Table 2 depicts trends of BNP levels in both groups at three-point intervals. The statistical difference between the two groups was not significant (P > 0.2, P > 0.35 and P > 0.5, respectively). When the trend of BNP levels with respect to time was considered, it was seen that the levels increased significantly in both groups as time progressed (P < 0.01), but the difference between the two groups was not significant (P > 0.2) [Table 2]. In this prospective study of 150 patients, the baseline B natriuretic peptide (BNP) levels correlated with the degree of cyanosis in the two groups. We observed in Group I BNP level of 215 pg/mL with a sensitivity of 90% and specificity of 95%, post-induction (T1), BNP level of 255 pg/mL with a sensitivity of 90% and specificity of 90% at T2 time and after the termination of CPB, and BNP level of 301.47 ± 60.77 pg/mL with a sensitivity of 85% and specificity of 40%. 48 h post-surgery depicted higher mortality among the patients as depicted by the following receiver operating characteristic (ROC) with AUC 0.82 with a 95% confidence interval (CI) of 0.72–0.92 was observed in the 2 groups. Atrial and ventricular fibrillation were common in group 2. A positive correlation was observed (although weak) between BNP at 48 h with secondary outcome post-operative measures of inotrope, ICU stay, and ventilation duration [Table 3].
| BNP levels (pg/mL) | Group 1 | Group 2 | P-value |
|---|---|---|---|
| T1 | 213.73±55.30 | 227.87±76.63 | NS |
| T2 | 260.65±57.93 | 270.67±77.18 | NS |
| T3 | 301.47±60.77 | 309±80.61 | NS |
S.D.: Standard deviation, BNP: B natriuretic peptide.
| Parameters | Group 1 | Group 2 | P-value |
|---|---|---|---|
| ICU stay (days) | 5.1±1.8 | 5.2±1.73 | NS |
| Duration of inotropes (days) | 3.9±1.4 | 3.2±1.5 days | NS |
| Duration of ventilation (hours) | 16.4±6.4 | 16.7±7 h | NS |
| Mortality (n) | 3 | 8 | NS |
| Rhythm disturbances (AF, VF) (n) | 33 | 45 | NS |
ICU: Intensive care unit, S.D.: Standard deviation, AF: Atrial fibrillation, VF: Ventricular fibrillation
Mortality
There was a mortality of eleven patients – three from group I and eight from group 2.
Endothelin
The average Endothelin levels in Group 1 post-intubation, after the termination of CPB, and 48 hours after surgery were 3.04 ± 2.90, 9.92 ± 7.18, and 6.11 ± 4.63 pg/mL, respectively, and in group 2, they were 2.75 ± 2.25, 8.31 ± 6.20 and 5.70 ± 4.55, respectively. There was a positive correlation between the Endothelin levels at 48 h after bypass and post-operative secondary outcome measures of duration of ICU stay as well.
We observed the Endothelin levels taken at 48 hours postoperatively were higher than 315 pg/mL, and the patients had a high probability of mortality. The following ROC curve in Figure 1 depicts the relationship between Endothelin levels taken 48 hours postoperatively and mortality rate, with a sensitivity of 66.7% and a specificity of 59%. Table 4 displays the average Endothelin levels in both the groups of patients who had died as well as in those who had survived.
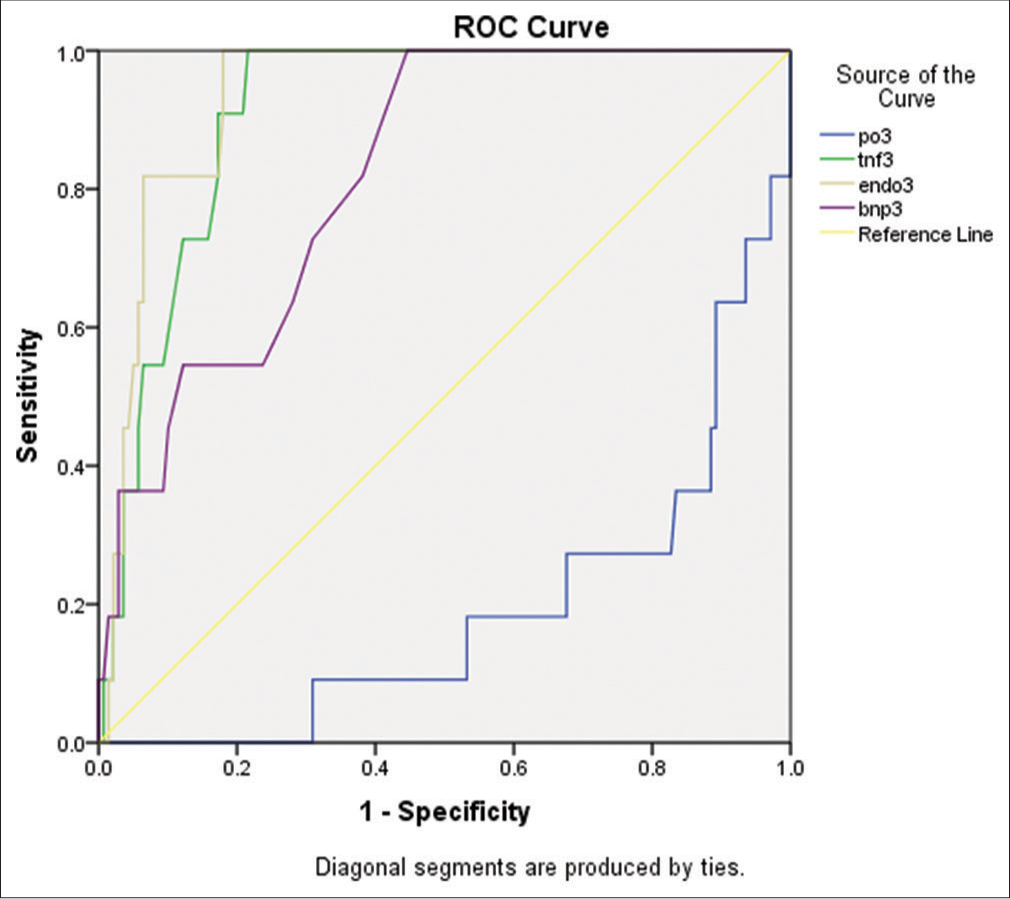
- Receiver operating characteristic (ROC) curve of 4 biomarkers tumor necrosis factor-alpha, Brain natriuretic peptide (BNP) Endothelin, and base excess.
| group | mean Endothelin levels (pg/ml) |
|---|---|
| Survivors (n=139) | 4.2±0.9 |
| Non-survivors (n=11) | 8.9±1.3 |
TNF-α
At 24 hours post-CPB (T3), there was a significant (P < 0.001) decrease in serum concentration of TNF-α in both the groups when compared with values immediately after CPB (T2), although they were still significantly (P < 0.001) higher than the pre-CPB measurement (T1).
Correlation between the duration of bypass and TNF-α levels measured 24 h post-CPB showed a significant correlation (P = 0.06) in Group I patients. Mortality in Group II correlated significantly (P < 0.05) with pre-CPB levels of TNF-α [Table 5].
| Time | TNF-α levels (pg/mL) | |||
|---|---|---|---|---|
| Group I | P-value | Group II | P-value | |
| Before CPB (T1) | 4.1±3.6 | - | 4.2±3.9 | - |
| After CPB (T2) | 11.3±11.1 | <0.001 | 11.6±10.2 | <0.001 |
| 24 h after CPB (T3) | 6.3±5.8 | <0.001 | 7.1±5.2 | <0.001 |
CPB: Cardiopulmonary bypass, TNF-α: Tumor necrosis factor-α
PO2, base excess, and lactate
The trend analysis of PO2 was done by the Bonferroni method of multiple comparisons in group 1, which showed a statistically significant difference of PO2 measured at baseline (T1) 81.74 ± 21.87 in group 1 and 83.72 ± 26.40 in group 2 with that after CPB (T2) and at 24 h in ICU (T3) (P < 0.001) and there was no statistical difference of between PO2 measured at CPB (T2) and at 24 h in ICU (T3) (P = 1.00) which shows that PO2 which increased after CPB maintained similar trends in ICU also. Such a similar increase in PO2 was also seen in both pediatric and adult patients in both groups.
The difference in base excess was found significant with time between baseline values (T1) and that measured at T2 and T3 (P = 0.001) but not between T2 and T3 (P = 0.8). The base excess trend comparison was similar between Group 1 and Group 2 [Table 6].
| Variable | Group 1 | Group 2 | P-value |
|---|---|---|---|
| PO2 (T1) | 81.74±21.87 | 83.72±26.40 | 0.617 |
| PO2 (T2) | 117.42±63.68 | 119.06±73.11 | O.883 |
| PO2 (T3) | 119.57±6.33 | 122.26±96.87 | 0.863 |
| Base excess (T1) | 5.94±1.19 | 5.76±1.22 | 0.382 |
| Base excess (T2) | 5.02±2.26 | 5.03±1.63 | 0.990 |
| Base excess (T3) | 4.42±3.16 | 4.50±3.000 | 0.880 |
| Lactate (T1) | 1.60±0.44 | 1.74±0.59 | 0.121 |
| Lactate (T2) | 3.77±0.61 | 3.23±0.66 | 0.578 |
| Lactate (T3) | 2.26±0.83 | 2.25±0.79 | 0.954 |
PO2: Pressure of oxygen
The lactate value raised from its baseline 1.60 ± 0.44 in group 1 and 1.74 ± 0.59 in group 2, measured at T1 to 3.77 ± 0.61 in group 1 and 3.23 ± 0.66 in group 2 after CPB and, then, it was seen as reduced lactate levels at T3 (2.26 ± 0.83 and 2.25 ± 0.79) in group 1 and group 2, respectively. The mean initial PO2 (T1) was similar in survivors, 83.68 ± 24.561, compared to non-survivors 70.69 ± 14.81 (P = 0.086). In survivors, there was an adequate PO2 for cellular perfusion after surgical repair, 121.31 ± 69.95 (T2), in comparison to non-survivors who had an average PO2 of 79.40 ± 16.26 (P < 0.001). In non-survivors at 24 h in ICU (T3), there was a further decrease of PO2 seen to a mean value of 59.86 ± 15.09 (T3). In survivors, PO2 seen was 125.86 ± 95.09 (P < 0.001) [Table 6].
The base excess values were similar initially (T1), measuring 5.86 ± 1.21 and 5.79 ± 1.20 in survivors and non-survivors, respectively (P = 0.086), and after surgical repair, measuring 5.03 ± 2.01 and 4.90 ± 1.34 in survivors and non-survivors, respectively (P = 0.774). However, in non-survivors, the value decreased to 2.8 ± 4.27 in comparison to 5.04 ± 2.06 in survivors, and there was a statistically significant difference between the post-operative base excess values (P < 0.001) [Table 7].
| Variable | Survivor (n=139) |
Non-survivor (n=11) | P-value |
|---|---|---|---|
| PO2 (T1) | 83.68±24.561 | 70.69±14.81 | 0.086 |
| PO2 (T2) | 121.31±69.95 | 79.40±16.26 | <0.001 |
| PO2 (T3) | 125.86±95.09 | 59.86±15.09 | <0.001 |
| Base excess (T1) | 5.86±1.21 | 5.79±1.20 | 0.854 |
| Base excess (T2) | 5.03±2.01 | 4.90±1.34 | 0.774 |
| Base excess (T3) | 5.04±2.06 | −2.8±4.27 | <0.001 |
| Lactate (T1) | 1.62±0.45 | 2.29±0.923 | <0.001 |
| Lactate (T2) | 3.20±0.63 | 3.26±0.7 | 0.753 |
| Lactate (T3) | 2.11±0.63 | 4.01±0.73 | <0.001 |
PO2: Pressure of oxygen
The TOF score with research done at All India Institute of Medical Sciences (AIIMS)
Scoring was based on four regression coefficients for biomarkers, namely, BNP, base excess, TNF-α, and Endothelin, and the method used was logistic regression (stepwise forward). We found that in increasing order, the four biomarkers, which were 7.89, 9.17, 21.47, and 32.9, respectively, for Endothelin, BNP, TNF-α, and base excess [Tables 8 and 9, Figure 1].
| Biomarkers | Significance | Odd ratio | 95% C.I. for odd ratio | |
|---|---|---|---|---|
| TNF-α | 0.021 | 21.469 | Lower | Upper |
| BNP | 0.065 | 9.175 | 0.868 | 96.954 |
| Endothelin | 0.097 | 7.886 | 0.688 | 90.343 |
| Base excess | 0.005 | 32.899 | 2.939 | 368.220 |
AIIMS: All India Institute of Medical Sciences, TNF-α: Tumor necrosis factor-alpha, C.I.: Confidence interval, TOF: Tetralogy of Fallot, BNP: B natriuretic peptide.
|
TNF-α: Tumor necrosis factor-alpha, TOF: Tetralogy of Fallot
Using the model score equation

In Tables 8 and 9, Figure 1, we observed that base excess has maximum contribution toward mortality, and the TNF-α score is next.
Summation of the above equation and from the values of multivariate logistic analysis of regression coefficient, there is a 97% predictability of mortality based (93.8–100%) on these four biomarkers as shown in the ROC curve [Figure 2].
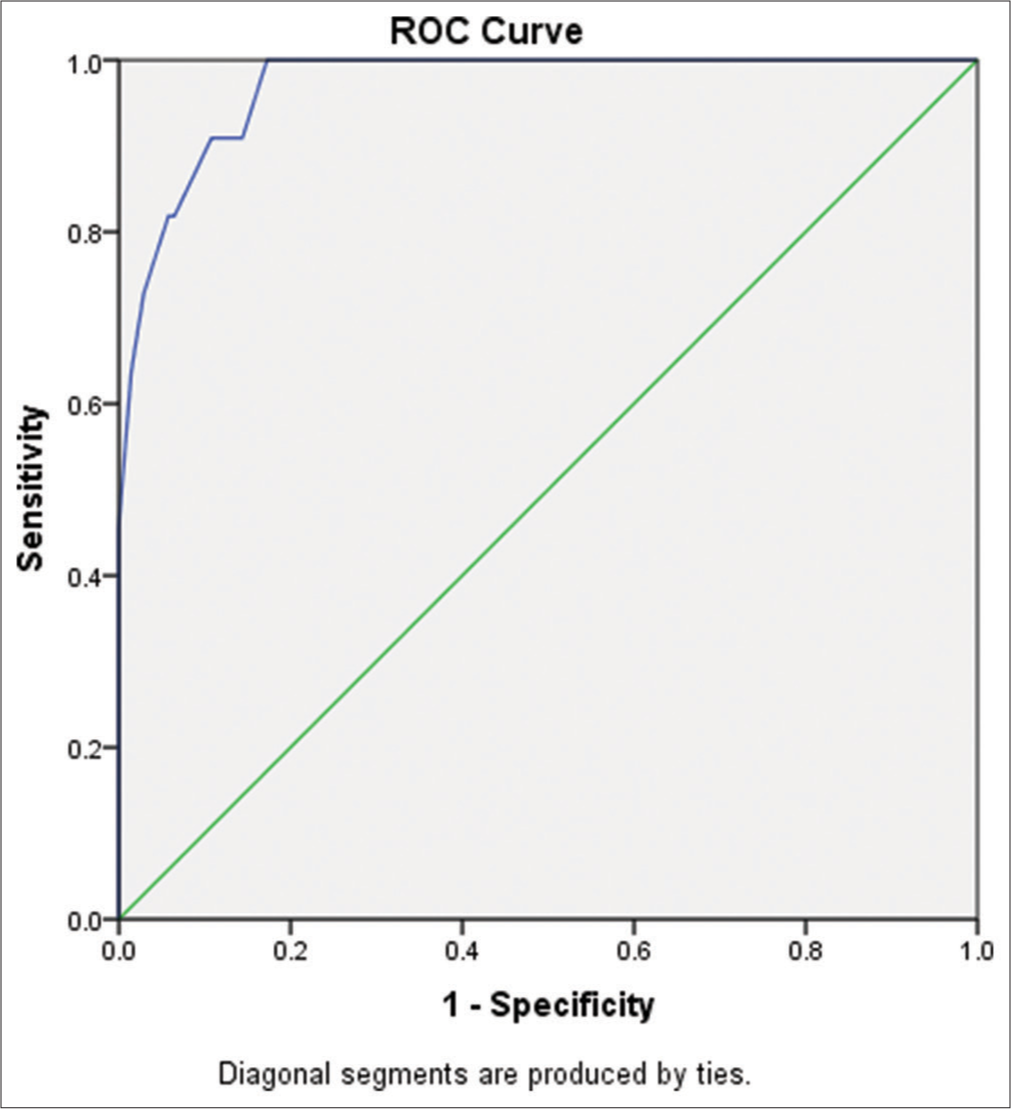
- Receiver operating characteristic (ROC) curve showing sensitivity and specificity for major four biomarkers used to prognosticate mortality in tetralogy of Fallot.
DISCUSSION
The study of most cardiac biomarkers in TOF is an enigma. The predictive value after the intracardiac repair of most cardiac biomarkers, such as BNP, has not been estimated extensively, and this indicates worsening postoperative outcomes.[11]
Endothelin interacts with other vasoactive substances, stimulating the release of Endothelin-derived relaxing factor, prostacyclin, and thromboxane A2 in the lung.[12]
In agreement with Mair et al.[13] and Berendes et al.,[14] the present study showed a trend of increase in most biomarker levels post-CPB due to the ischemia from aortic cross-clamping, which acts as a stimulant for the biomarker release.
Like our study, several studies have validated that high pre-operative BNP levels have been associated with a longer postoperative ICU stay, mechanical ventilation, and inotrope use.[11]
The increase occurs on early reperfusion after prolonged low-flow ischemia and the CPB might cause endothelial damage and induce the release of Endothelin (ET-1).[15] A similar rise was seen when the Endothelin levels were noted before and immediately after coming off the bypass in our study in both groups. The levels came down after 48 hours, probably due to the settling down of inflammation and the low flow state.
Mayyas et al.[16] correlated Endothelin levels to the development of atrial fibrillation. Similarly, in our study, the older age group had a higher incidence of atrial fibrillation.
This could be due to the advanced disease process with resultant ventricular dysfunction seen in the older age group and high Endothelin levels seen in TOF patients with ventricular fibrosis and dysfunction.[17]
Increased levels of TNF-α in cyanotic heart disease have been shown in many studies. Cyanotic patients had significantly elevated TNF-α levels (33% higher) than the healthy control population in a study done by Sharma et al.[18-20] Similarly, another study done by Qing et al. demonstrated higher TNF-α in cyanotic than acyanotic heart disease patients.[19] A wide interindividual variation in TNF-α was observed in our study, which could be attributed to some TOF patients having a genotype that has high TNF-α levels and thus more morbidity and mortality. Our study demonstrated significantly elevated TNF-α levels in the post-CPB period, which peaked post-protamine. Although levels decreased after 48 hours, they were still higher than the pre-operative value. This was in accordance with many previous studies in patients with congenital or acquired heart disease.[21,22]
Another interesting observation in our study was that the elevated TNF-α level correlated significantly with adverse clinical outcomes in the younger age group but not in Group II. Thus suggesting a similar rise in TNF-α levels might lead to a more severe inflammatory response in younger children. Lequier et al., in their study, showed an aggressive inflammatory response seen in children with cyanotic congenital heart disease (CCHD)[23,24], which may lead to gut ischemia due to low cardiac output syndrome.
This could be due to age-dependent differences in the inflammatory response, increased susceptibility of immature organ systems, and a larger extracorporeal circuit to patient size ratio resulting in greater susceptibility of younger patients to the damaging effect of CPB.
Oxygen delivery, hematocrit, and cardiac output determinants are all essential for cellular perfusion in TOF patients. Below a certain point, decreased oxygen delivery cannot be compensated for by an increased oxygen extraction, resulting in anaerobic metabolism and lactic acidosis. In comparison between survivors and non-survivors, an interesting observation was that in non-survivors, the PO2 rise was not sustained after CPB, and PO2 further decreased in ICU (P < 0.001). In our study, we found that PO2 in non-survivors was low as compared to the survivors and the cutoff of PO2 was 66.1, an ROC curve showing an AUC of 67%.[25] However, PO2 itself may not always reflect tissue oxygen utilization and may not be an effective marker of occult hypoperfusion in comparison to lactate.
Lactate production results from the cellular metabolism of pyruvate into lactate under anaerobic conditions.[26,27] In our study, the lactate value increased from its baseline measured at T1 to that after CPB, and then, it started to be cleared from the circulation, as shown by decreasing values measured at T3 in both group 1 and group 2.[28] Intragroup comparison of lactate value among survivors showed significant changes with time. Increased value was noted after CPB (T2) and decreased in ICU (T3) as lactate was cleared from circulation (P < 0.001). However, in non-survivors, lactate continued to show a significant increasing trend (P < 0.001). In our study, persistently elevated post-operative lactate is associated with increased mortality in both pediatric and adult patients undergoing ICR.
The arterial base deficit level is a calculated value based on the arterial pressure of carbon dioxide, PH, and serum HCO3 and represents the number of milliequivalents of the additional base that must be added to one liter of blood to normalize the PH.[29,30] In our study, it was found that base excess is a predictor of mortality in TOF patients with a high sensitivity of 89.9% and specificity of 90.9%. Hypoalbuminemia is a well-documented predictor of general surgical risk and frequently occurs in patients with CCHD. Low pre-operative serum albumin concentrations (<3.3 g/dL) can be used to identify and prognosticate a subset of cyanotic heart disease predisposed to additional surgical risk in TOF patients.[31] Proper perioperative care is only possible when a correct diagnosis is reached in situations causing a diagnostic dilemma between low oxygen saturation and clinical findings.[32]
Calculation of AIIMS TOF scoring was done using the regression coefficients for those biomarkers with maximum sensitivity and specificity. Based on scoring, the ROC curve revealed that AUC was 97% (P < 0.001) with (93.8–100) as 95% CI. The cutoff point revealed that if the score is <2.73, then it can differentiate well, with 96.9% as sensitivity and 89.2% as specificity, as shown in Figure 2. This model score classifies 94.7% correctly for depicting mortality in TOF patients.
The AIIMS TOF score
We found four biomarkers, the most sensitive and specific, with a score of 2.73. If the score is more than 2.73, then the chances of mortality are high, with a sensitivity of 96.9% and specificity of 89.2%. The area under the curve will be 97%, which is significant [Tables 8 and 9].
The normal inflammatory response seen post-ICR corrective surgery does show raised levels of inflammatory markers such as TNF-α, BNP, base excess, and Endothelin. This is due to complement activation of complements, neutrophils, arachidonic acid, kinins, cytokines (Interleukins6, 8, and 10), Endothelin, and platelet-activating factor, which we have not reported in our study. A comparison of each biomarker was done in our study with non-specific parameters such as hospital and ICU stay and duration of mechanical ventilation. We did not compare the biomarkers with targeted anti-inflammatory treatments. This limitation should be overcome in future studies, which should be able to showcase a high risk of inflammation post-correction.
Limitations
What is the value of these biomarkers after the management of patients? Is this a dilemma, as most cardiac centers do not use BNP, albumin, TNF-α, or Endothelin on a routine basis? This remains a major limitation for most patients, even those with elevated levels; it can be argued that the measurement of cardiac biomarkers is probably of little benefit because postoperative cardiac patients are already receiving appropriate maximal therapy for secondary prevention of cardiac events.[33]
Including an imaging parameter like global longitudinal strain on transesophageal echocardiography (TEE) could have added greater value to post-operative right ventricular function as a more dynamic parameter along with PO2, lactate, and base excess for better post-operative management.[34]
CONCLUSION
Four biomarkers, namely, Endothelin, TNF-α, BNP, and base excess, were found to be highly sensitive and specific. Using these biomarkers, a score of 2.73 (the AIIMS TOF score) is considered in morbid patients post-ICR in the ICU. The chances of mortality are high, with a sensitivity of 96.9% and a specificity of 89.2%. Predicting morbidity and mortality in ICR patients should be considered using prognostic biomarkers, as cited above.
Acknowledgment
Dr. Guresh Kumar, Chief Statistician, Department of BioStatistics, AIIMS, New Delhi, for his help and guidance in preparing the manuscript and statistics.
Ethical approval
The authors declare that they have taken the Institutional Ethics Committee approval and the approval number is (Trial No-5/4/1-1/08- NCD-II).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Poonam Malhotra Kapoor and Dr. Minati Choudhury are on the editorial board of the journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
This project was funded by ICMR.
References
- B-type Natriuretic Peptide in Cardiovascular Disease. Lancet. 2003;363:316-22.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating Endotoxin and Tumor Necrosis Factor During Pediatric Cardiac Surgery. Crit Care Med. 1992;20:1090-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine Responses to Cardiopulmonary Bypass: Lessons Learned from Cardiac Transplantation. Ann Thorac Surg. 1997;63:269-7.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin-10 Release Related to Cardiopulmonary Bypass in Infants Undergoing Cardiac Operations. J Thorac Cardiovasc Surg. 1996;111:545-53.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine Response in Children Undergoing Surgery for Congenital Heart Disease. Pediatr Cardiol. 2006;27:408-13.
- [CrossRef] [PubMed] [Google Scholar]
- The Relationship between Inflammatory Activation and Clinical Outcome after Infant Cardiopulmonary Bypass. Anesth Analg. 2010;111:1244-51.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of Acute Phase Response after Cardiopulmonary Bypass by Immunomodulation. Ann Thorac Surg. 1993;55:389-94.
- [CrossRef] [PubMed] [Google Scholar]
- Elevation of Cytokines During Open Heart Surgery with Cardiopulmonary Bypass: Participation of Interleukin 8 and 6 in Reperfusion Injury. Can J Anaesth. 1993;40:1016-21.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor Necrosis Factor and Clinical and Metabolic Courses after Cardiac Surgery in Children. J Thorac Cardiovasc Surg. 2002;124:991-8.
- [CrossRef] [PubMed] [Google Scholar]
- What is the Normal Range for N-terminal Pro-brain Natriuretic Peptide? How Well does this Normal Range Screen for Cardiovascular Disease? Eur Heart J. 2005;26:2269-76.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of B-type Natriuretic Peptide and Left Ventricular Dysfunction in Patients with Constrictive Pericarditis Undergoing Pericardiectomy. Ann Card Anaesth. 2010;13:123-9.
- [CrossRef] [PubMed] [Google Scholar]
- Is Endothelin Gene Polymorphism Associated with Postoperative Atrial Fibrillation in Patients Undergoing Coronary Artery Bypass Grafting? Ann Card Anaesth. 2017;20:341-7.
- [CrossRef] [PubMed] [Google Scholar]
- Augmented Release of Brain Natriuretic Peptide During Reperfusion of the Human Heart after Cardioplegic Cardiac Arrest. Clin Chim Acta. 1997;261:57-68.
- [CrossRef] [PubMed] [Google Scholar]
- A-type and B-type Natriuretic Peptides in Cardiac Surgical Procedures. Anesth Analg. 2004;98:11-9.
- [CrossRef] [PubMed] [Google Scholar]
- Increased Plasma Levels of Endothelin-1 after Cardiopulmonary Bypass in Patients with Pulmonary Hypertension and Congenital Heart Disease. J Thorac Cardiovasc Surg. 1993;106:473-8.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma Endothelin-1 Levels are Increased in Atrial Fibrillation Patients with Hyperthyroidism. PLoS One. 2018;13:e0208206.
- [CrossRef] [PubMed] [Google Scholar]
- Perioperative Endothelin Levels in Patients Undergoing Intracardiac Repair for Tetralogy of Fallot. J Card Surg. 2014;29:670-7.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated Circulating Levels of Inflammatory Cytokines and Bacterial Endotoxin in adults with Congenital Heart Disease. Am J Cardiol. 2003;92:188-93.
- [CrossRef] [PubMed] [Google Scholar]
- Intramyocardial Synthesis of Pro-and Anti-inflammatory Cytokines in Infants with Congenital Cardiac Defects. J Am Coll Cardiol. 2003;41:2266-74.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor Necrosis Factor Alpha Influences the Inflammatory Response after Coronary Surgery. Ann Thorac Surg. 2006;81:132-7.
- [CrossRef] [PubMed] [Google Scholar]
- Perioperative Levels of Tumor Necrosis Factor-α Correlate with Outcomes in Children and adults with Tetralogy of Fallot Undergoing Corrective Surgery. World J Pediatr Congenit Heart Surg. 2014;5:38-46.
- [CrossRef] [PubMed] [Google Scholar]
- Pro-inflammatory Cytokine TNF α as A Prognostic Marker in Children with Tetralogy of Fallot Undergoing Intracardiac Repair. J Card Crit Care. 2023;7:95-9.
- [Google Scholar]
- Preoperative and Postoperative Endotoxemia in Children with Congenital Heart Disease. Chest. 2000;117:1706-12.
- [CrossRef] [PubMed] [Google Scholar]
- Serum TNF-α Levels in Children with Congenital Heart Disease Undergoing Cardiopulmonary Bypass: A Cohort Study in China and a Meta-analysis of the Published Literature. J Clin Lab Anal. 2017;31:e22112.
- [CrossRef] [PubMed] [Google Scholar]
- Lactate, Endothelin, and Central Venous Oxygen Saturation as Predictors of Mortality in Patients with Tetralogy of Fallot. Ann Card Anaesth. 2016;19:269-76.
- [CrossRef] [PubMed] [Google Scholar]
- Serum Lactate and A Relative Change in Lactate as Predictors of Mortality in Patients with Cardiogenic Shock-Results from the Cardshock Study. Shock. 2020;53:43-9.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome with High Blood Lactate Levels During Cardiopulmonary Bypass in adult Cardiac Operation. Ann Thorac Surg. 2000;70:2082-6.
- [CrossRef] [PubMed] [Google Scholar]
- Is There any Correlation between PO2, Base Excess and Lactate. Biomarkers. 2018;6.1:108-11.
- [Google Scholar]
- The Role of Blood Lactate Clearance as A Predictor of Mortality in Children Undergoing Surgery for Tetralogy of Fallot. Ann Card Anaesth. 2016;19:217-24.
- [CrossRef] [PubMed] [Google Scholar]
- Basic Arterial Blood Gas Biomarkers as a Predictor of Mortality in Tetralogy of Fallot Patients. Ann Card Anaesth. 2017;20:67-71.
- [CrossRef] [PubMed] [Google Scholar]
- Serum Albumin Perturbations in Cyanotics after Cardiac Surgery: Patterns and Predictions. Ann Card Anaesth. 2016;19:300-5.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic Dilemma: Low Oxygen Saturation During Cardiac Surgery. Ann Card Anaesth. 2017;20:262-4.
- [CrossRef] [PubMed] [Google Scholar]
- Do We Really Need Another Biomarker to Diagnose Myocardial Infarction after Coronary Artery Bypass Graft Surgery? Anesth Analg. 2010;111:1086-7.
- [CrossRef] [PubMed] [Google Scholar]
- Ventricular Function and Biomarkers in Relation to Repair and Pulmonary Valve Replacement for Tetralogy of Fallot. Open Heart. 2023;10:e002238.
- [CrossRef] [PubMed] [Google Scholar]






