Translate this page into:
Pro-inflammatory Cytokine TNF-α as a Prognostic Marker in Children with Tetralogy of Fallot Undergoing Intracardiac Repair
*Corresponding author: Rashmi Singh, Department of Cardiac Anaesthesia and Critical Care, AIIMS, Delhi, India. rashmisingh5509@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singh R, Prakash M, Mujahid OM, Badge M, Choudhury M. Pro-inflammatory cytokine TNF-α as a prognostic marker in children with tetralogy of Fallot undergoing intracardiac repair. J Card Crit Care TSS 2023;7:95-9.
Abstract
Objectives:
Patients with Tetralogy of Fallot (TOF) undergoing corrective surgery experience a vast majority of pulmonary, cardiac, and renal dysfunction due to the effect of cardiopulmonary bypass (CPB). Tumor necrosis factor-α (TNF-α) is a pro-inflammatory cytokine that has been shown to play a major role in systemic inflammatory response syndrome secondary to CPB. The rise of level of inflammatory mediator may serve as a clue for post-operative organ dysfunction and may determine the post-operative course of the patient. They may enable us to detect morbidity earlier, thereby taking steps to decrease the same. We have done this study with the aim to evaluate changes in serum concentration of TNF-α in patients with TOF undergoing intracardiac repair (ICR) on CPB and correlate changes in its level with correlate them with clinical outcome and mortality.
Material and Methods:
TOF patients divided into two groups (< and >15 years) after approval from the ethics committee and written informed consent were enrolled in this prospective and observational study with ICR on CPB. Routine anesthesia and CPB as per AIIMS protocol were performed on all the patients. Routine arterial blood gas, heart rate, mean arterial pressure, temperature, and urine output monitoring were done in all cases. Arterial blood samples were collected at three time intervals – T1, T2, and T3 and processed for biomarker level detection by Enzyme-linked immunosorbent assay method. Appropriate statistical analysis was done for the correlation between biomarkers and clinical variables.
Results:
There were no significant differences between the two groups in terms of gender distribution, CPB time, and aortic cross-clamp time. Duration of mechanical ventilation, inotropes use, intensive care unit stay, and hospital stay were all significantly prolonged (P < 0.001) in Group 1 (age <15 years) when compared to Group II (age more than 15 years).
Conclusion:
Level of TNF-α correlated significantly with duration of mechanical ventilation, ionotrope use, intensive care unit stay, and hospital stay in children <15 years. However, a larger study population is required to further prove this hypothesis.
Keywords
Tumor necrosis factor-α
Tetralogy of Fallot
Prognostic marker
CPB
INTRODUCTION
The most common cyanotic congenital heart disease (CHD) Tetralogy of Fallot (TOF) causes chronic tissue hypoxia and compensatory polycythemia. Low flow state, microvascular thrombosis, and chronic ischemia make these subsets particularly prone to ischemia-reperfusion injury post total correction. Cardiopulmonary bypass (CPB) induces a host of inflammatory responses leading to myocardial, pulmonary, and renal dysfunction. The effect of CPB-related hemodynamic perturbations is more pronounced in children due to immature myocardium and pulmonary system. The rise of level of inflammatory mediator may serve as a clue for postoperative organ dysfunction and may determine the post-operative course of the patient. They may enable us to detect morbidity earlier, thereby taking steps to decrease the same.
Tumor necrosis factor-α (TNF-α) is a pro-inflammatory cytokine that has been shown to play a major role in systemic inflammatory response syndrome secondary to CPB. Various studies have been done to prove the correlation between CPB and level of TNF-α but they have conflicting results. While some studies suggest a rise in TNF-α,[1-3] some describe no changes[4,5] while a decrement in its level has also been shown in some studies.[6-8] Major limitation of these studies was the small sample size and diagnostic variability.
The aim of our study is to evaluate changes in serum concentration of TNF-α in patients with TOF undergoing intracardiac repair (ICR) on CPB and correlate changes in its level with correlate them with clinical outcome and mortality.
MATERIAL AND METHODS
After approval from the Institute Ethics Committee and written and informed consent, a prospective and observational study was conducted on 150 TOF patients undergoing elective ICR on CPB. The patients were divided into two groups. Group I included patients <15 years of age whereas Group II included patients more than 15 years of age. Patients with preexisting congestive cardiac failure, coagulopathy, renal failure (serum creatinine >2 mg/dL, anuria, or oliguria requiring dialysis), immune or central nervous system dysfunction, local or systemic infection, or inflammation (fever, leukocytosis, tachycardia, or tachypnea), on immunosuppressive or anti-inflammatory therapy, were not included in the study.
Anesthetic and surgical management were standardized in all patients. Anesthesia was induced with ketamine (1–2 mg/kg), fentanyl (2–3 μg/kg), and rocuronium-bromide (0.8–1 mg/kg). Maintainance was done with sevoflurane (0.5–1%) in an oxygen-air mixture with intermittent doses of fentanyl, midazolam, and vecuronium. Blood gas analysis and activated clotting time were performed intraoperatively, at half-hourly intervals. At the end of the surgery, the sternum was closed, and patients were shifted to the intensive care unit (ICU) for elective ventilation. The intravenous infusion of injection dopamine was started in all patients, and if required, dobutamine was added to maintain hemodynamics.
Serum TNF-α levels were measured using Quanttikine Human TNF-α Immunoassay, a solid phase Enzyme-linked immunosorbent assay (R and D systems, US). The minimum detectable dose of TNF-α ranges from 0.5 to 5.5 pg/mL.
Blood samples were collected at the following time intervals.
T1 – After induction (baseline value)
T2 – 20 min after protamine administration
T3 – 48 h after surgery in the ICU.
Intraoperative CPB duration was noted. Once the patients were shifted to the ICU, the duration of postoperative ventilation, inotropic use, and stay in ICU was recorded.
Statistical analysis
The data were analyzed in SPSS software version 20 (IBM corporation, USA). The qualitative data were compared applying the Chi-square test. The quantitative data were analyzed using Student’s t-test and Wilcoxon-Rank sum, wherever applicable. To observe the change over a period of time in various biomarkers, repeated measures analysis was applied by the Bonferroni method. The receiver operating characteristic analysis was used to find area under the curve and cutoff values of biomarkers for predicting mortality.
RESULTS
A total of 150 patients were included in the study. Demographic data and perioperative parameters of the study participants in the two groups are shown in [Table 1]. There was no significant difference between the two groups in terms of gender distribution, CPB time and aortic cross-clamp time. Duration of mechanical ventilation, inotropes use, intensive care unit stay, and hospital stay were all significantly prolonged (P < 0.001) in Group 1 (age <15 years) when compared to Group II (age more than 15 years). There were 11 in-hospital deaths (overall mortality of 7.3%). Although mortality was higher in Group II, it was not statistically significant (P > 0.05) when compared with Group I.
| Parameter | Group I n=75 median (range) | Group II n=75 median (range) | P-value |
|---|---|---|---|
| Age (years) | 4 (0.11–14) | 31 (16–55) | - |
| Weight (kg) | 12 (2–56) | 48 (30–85) | - |
| Gender | Females 44% Males 56% |
Females 36% Males 64% |
NS |
| CPB time (minutes) | 90 (76–104) | 87 (73–104) | NS |
| Clamp time (minutes) | 41 (29–75) | 38 (31–78) | NS |
| Mechanical ventilation (hours) | 17 (8–20) | 10 (6–20) | P<0.001 |
| Inotrope use (days) | 4 (1–5) | 2 (1–5) | P<0.001 |
| ICU stay (days) | 4 (2–6) | 3 (2–7) | P<0.001 |
| Hospital stay (days) | 8 (4–11) | 6 (4–10) | P<0.001 |
| Mortality (%) | 2.7 (n=3) | 5.3 (n=8) | NS |
CPB: Cardiopulmonary bypass, ICU: Intensive care unit
TNF-α levels and CPB
TNF-α levels in both the groups at various time intervals are shown in [Table 2].
| Time | TNF-α levels (pg/mL) | |||
|---|---|---|---|---|
| Group I | P-value | Group II | P-value | |
| Before CPB (T1) | 4.1±3.6 | - | 4.2±3.9 | - |
| After CPB (T2) | 11.3±11.1 | <0.001 | 11.6±10.2 | <0.001 |
| 24 h after CPB (T3) | 6.3±5.8 | <0.001 | 7.1±5.2 | <0.001 |
TNF-α: Tumor necrosis factor-α, CPB: Cardiopulmonary bypass
There were no significant (P > 0.05) intergroup differences in the serum levels of TNF-α at the 3-time points in the study. TNF-α levels were significantly (P < 0.001) elevated in post-CPB period in both groups. At 24-h post-CPB (T3), there was a significant (P < 0.001) decrease in serum concentration of TNF-α in both the groups, when compared with values immediately after CPB (T2), although they were still significantly (P < 0.001) higher than the pre-CPB measurement (T1).
TNF-α levels in relation to clinical outcome
Duration of hospital stay in Group 1 patients correlated significantly with serum TNF-α measurement in the preCPB period (P < 0.05) [Figure 1], immediate post-CPB period (P < 0.001) [Figure 2], and 24-h post-CPB (P < 0.001) [Figure 3]. Serum TNF-α concentration immediately after CPB showed a tendency toward significant correlation with the duration of CPB [Figure 4] as well as the duration of mechanical ventilation [Figure 5] in group 1. Correlation between the duration of bypass and TNF-α levels measured 24-h post-CPB showed a tendency toward significance (P = 0.06) in Group I patients. In Group II, no correlation existed between any clinical variable and TNF-α measurements made at any point in time. Serum TNF-α levels measured before bypass showed a tendency toward significant correlation with mortality in Group I (P = 0.09). Mortality in Group II correlated significantly (P < 0.05) with pre-CPB levels of TNF-α in Group II.
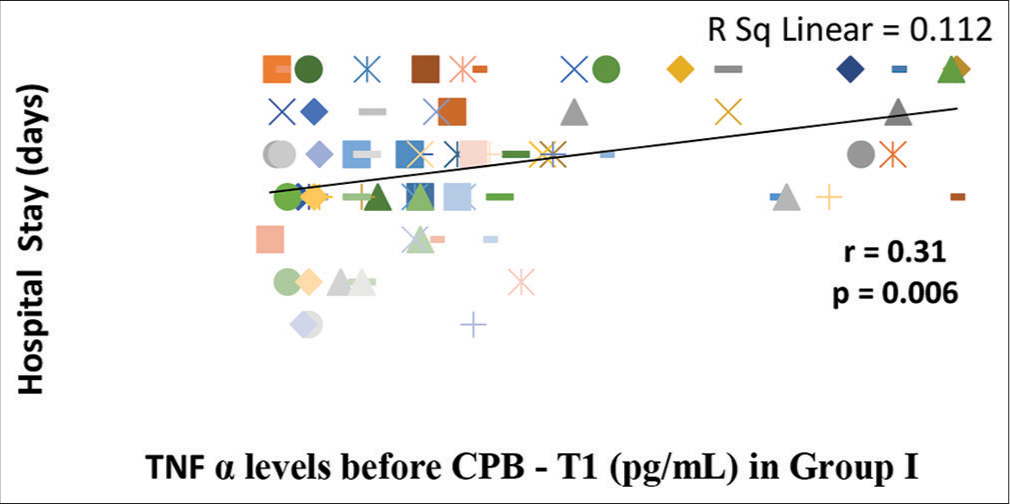
- Correlation of serum tumor necrosis factor-α (TNF-α) levels before cardiopulmonary bypass (CPB) (T1) with duration of hospital stay in Group I. There is significant correlation (P = 0.006) between pre bypass serum TNF-α levels and duration of hospital stay as analyzed by Spearman Rank Correlation Coefficient (r). The line represents the line of best fit.
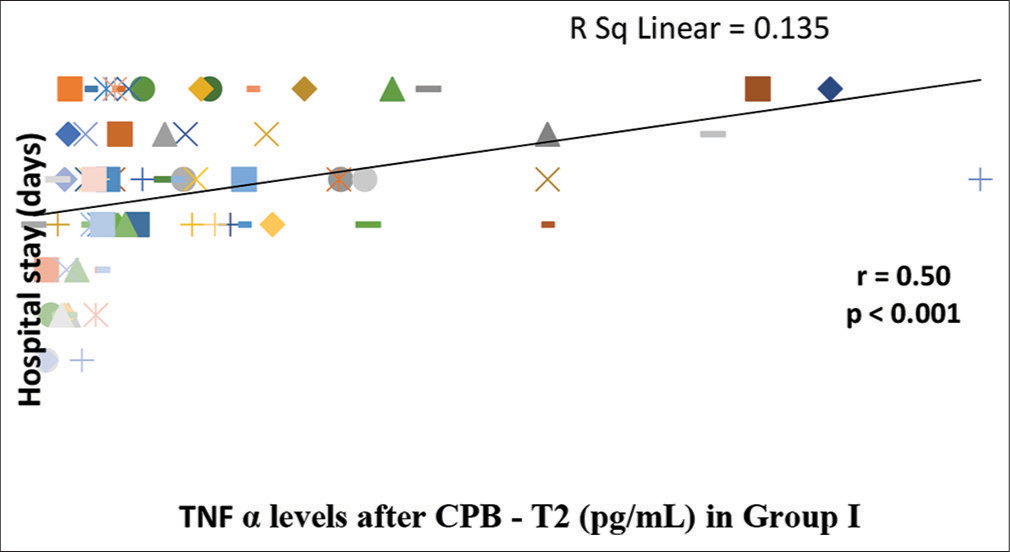
- Correlation of serum tumor necrosis factor-α (TNF-α) levels after cardiopulmonary bypass (CPB) (T2) with duration of hospital stay in Group I. There is a significant correlation (P < 0.001) between post-bypass serum TNF α levels and duration of hospital stay as analyzed by Spearman Rank Correlation Coefficient (r). The line represents the line of best fit.
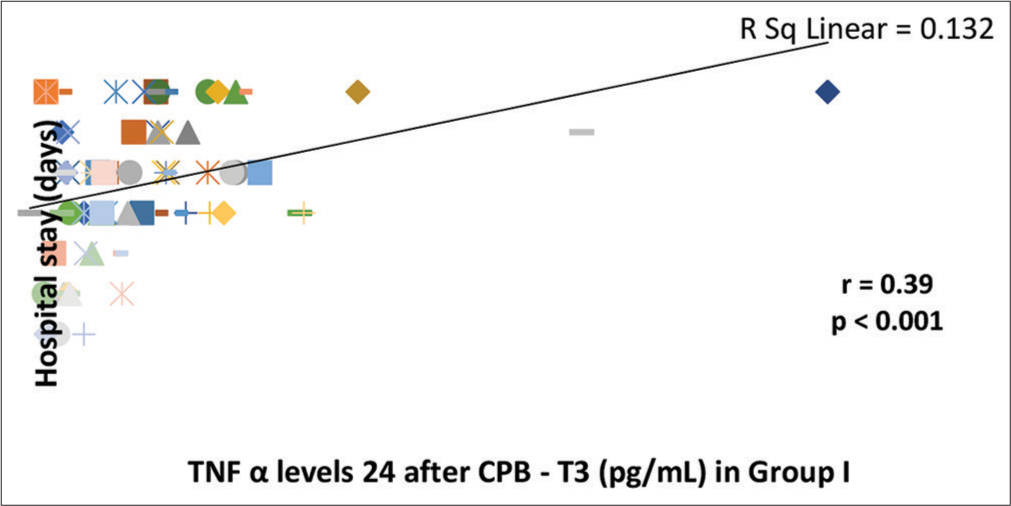
- Correlation of serum tumor necrosis factor-α (TNF-α) levels 24 h after cardiopulmonary bypass (CPB) (T3) with duration of hospital stay in Group I. There is a significant correlation (P < 0.001) between serum TNF-α levels 24 h after CPB and duration of hospital stay as analyzed by Spearman Rank Correlation Coefficient (r). The line represents the line of best fit.
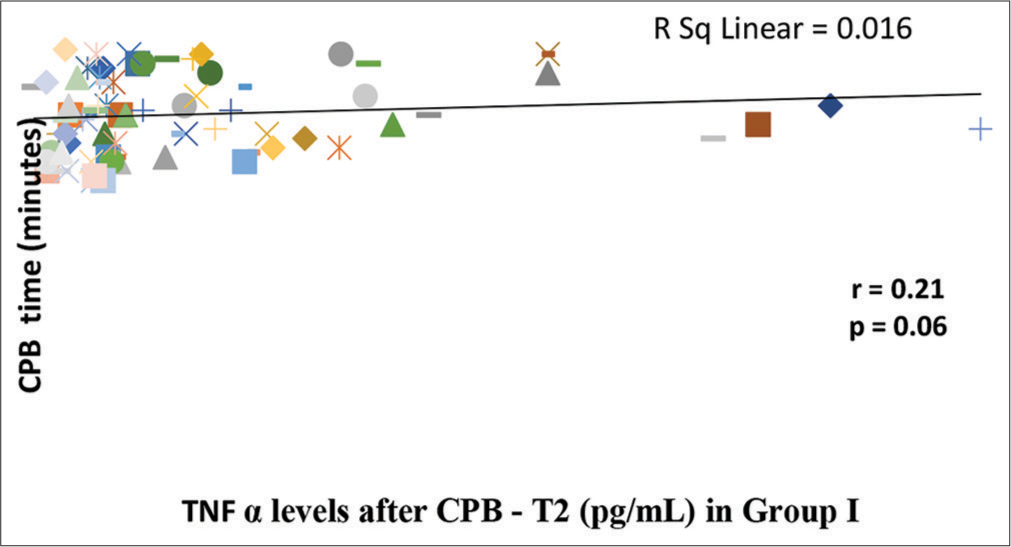
- Correlation of serum tumor necrosis factor-α (TNF-α) levels after cardiopulmonary bypass (CPB) (T2) with duration of CPB in Group I. There is tendency toward significant correlation (P = 0.06) between post-bypass serum TNF-α levels and duration of CPB as analyzed by Spearman Rank Correlation Coefficient (r). The line represents the line of best fit.
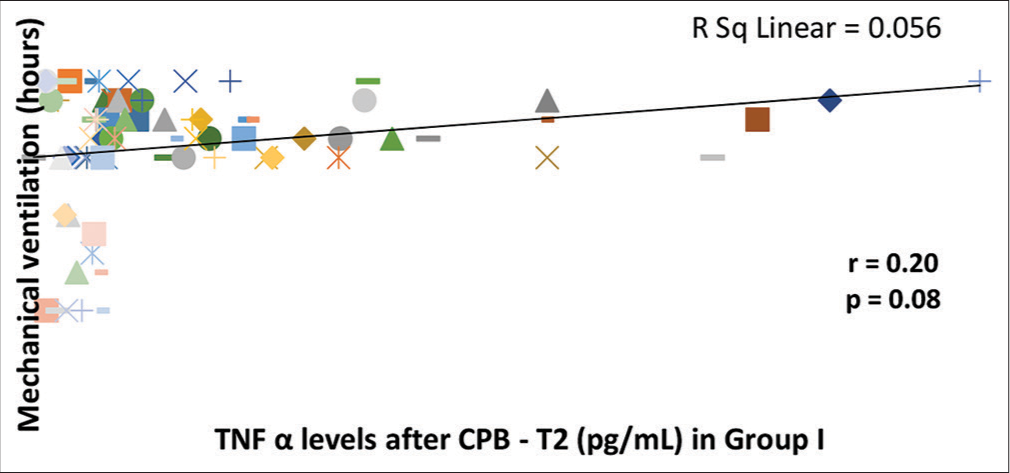
- Correlation of serum tumor necrosis factor-α (TNF-α) levels after cardiopulmonary bypass (CPB) (T2) with duration of mechanical ventilation in Group I. There is a tendency toward significant correlation (P = 0.08) between serum TNF-α levels after CPB (T2) and duration of mechanical ventilation as analyzed by Spearman Rank Correlation Coefficient (r). The line represents the line of best fit.
DISCUSSION
Till date, many studies have been published showing trends of TNF-α with CPB, but limited by small sample size and variable results. The highlight of our study is the large sample size (n = 150), in which perioperative trends of TNF-α and its correlation with clinical outcomes have been studied.
Another highlight of this study is the division of the study population into two groups of age less than and more than 15, as an inflammatory response is thought to be varied across the age spectrum.
TNF-α is a powerful inflammatory mediator, produced by activated monocytes and mononuclear phagocytes. TOF patients undergoing corrective surgery are exposed to various stimuli which initiate an inflammatory response. These insults can be due to general anesthesia, surgical wounds, CPB induced, ischemia-reperfusion, and protamine administration. All of these factors eventually become a nidus for an inflammatory surge.
Increased levels of TNF-α in cyanotic have been shown in many studies. Fifty-two cyanotic patients were found to have significantly elevated TNF-α levels (33% higher) than the healthy control population in a study done by BT Sharma et al.[9] Similarly, another study done by Qing et al. demonstrated higher TNF-α in cyanotic than acyanotic.[10] We observed a wide interindividual variation in TNF-α in our study. This could be attributed to Bittar et al. in their study on TNF-α response after coronary surgery.[11] They concluded that the AA genotype had higher circulating levels of TNF-α and this genotype has a less favorable outcome. Our study demonstrated significantly elevated TNF-α levels in a post-CPB period which peaked post-protamine. Although levels decrease after 48 h, they were still higher than the preoperative value. This was in accordance with many previous studies in patients with congenital or acquired heart disease.[12,13]
We noticed that despite standardized surgical management and no difference in the CPB and aortic cross-clamp time, Group I had significantly prolonged (P < 0.001) duration of mechanical ventilation, inotrope use, intensive care unit stay, and hospital stay. This could be because of age-dependent differences in the inflammatory response, increased susceptibility of immature organ systems, and a larger extracorporeal circuit to patient size ratio resulting in greater susceptibility of younger patients to the damaging effect of CPB.
Another interesting observation in our study was that the elevated TNF-α level correlated significantly with adverse clinical outcomes in the younger age group but not in Group II. Thus, suggesting a similar rise in TNF-α levels might lead to a more severe inflammatory response in younger children. Lequier et al. in their study showed the role of endotoxemia in the aggressive inflammatory response seen in children with CHD.[14] They suggested that low cardiac output in these children may cause gut ischemia leading to the translocation of bacterial endotoxin. Mou et al. also reported elevated endotoxin and TNF-α in children with CHD.[15] Ghobrel et al. using transcriptomic analysis of patients with TOF concluded that chronic hypoxia in cyanotic children induced the expression of genes associated with myocardium contractility and function therefore leading to adverse outcomes.[16]
TNF-α may act as an important biological indicator for monitoring the efficacy of CPB in children. The study performed by Song et al. compared TNF levels at various time points during on-pump cardiac surgery and concluded that CPB may attenuate inflammatory reactions compared to off-pump surgery.[17] Furthermore, they showed a marked decline after 24 h of CPB, when compared with the TNF-α level before the operation and at the end of the operation, suggesting that TNF-α may act as an important biological indicator for monitoring the efficacy of CPB in children with CHD.
Limitation
TNF-α levels no doubt are an important inflammatory mediator but the whole of the inflammatory spectrum consists of multiple other mediators such as cytokines, complement activation, neutrophil activation, platelets mediated activity, and endothelin. We have just studied one parameter among the whole of inflammatory mediators.
CONCLUSION
We demonstrated rise of TNF-α levels in patient with TOF undergoing ICR on CPB. Level of TNF-α correlated significantly with duration of mechanical ventilation, ionotrope use, ICU stay, and hospital stay in children <15 years. However, a larger study population is required to further prove this hypothesis.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Circulating endotoxin and tumor necrosis factor during pediatric cardiac surgery. Crit Care Med. 1992;20:1090-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine responses to cardiopulmonary bypass: Lessons learned from cardiac transplantation. Ann Thorac Surg. 1997;63:269-7.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin-10 release related to cardiopulmonary bypass in infants undergoing cardiac operations. J Thorac Cardiovasc Surg. 1996;111:545-53.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine response in children undergoing surgery for congenital heart disease. Pediatr Cardiol. 2006;27:408-13.
- [CrossRef] [PubMed] [Google Scholar]
- The relationship between inflammatory activation and clinical outcome after infant cardiopulmonary bypass. Anesth Analg. 2010;111:1244-51.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of acute phase response after cardiopulmonary bypass by immunomodulation. Ann Thorac Surg. 1993;55:389-94.
- [CrossRef] [PubMed] [Google Scholar]
- Elevation of cytokines during open heart surgery with cardiopulmonary bypass: Participation of interleukin 8 and 6 in reperfusion injury. Can J Anaesth. 1993;40:1016-21.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor necrosis factor and clinical and metabolic courses after cardiac surgery in children. J Thorac Cardiovasc Surg. 2002;124:991-8.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated circulating levels of inflammatory cytokines and bacterial endotoxin in adults with congenital heart disease. Am J Cardiol. 2003;92:188-93.
- [CrossRef] [PubMed] [Google Scholar]
- Intramyocardial synthesis of proand anti-inflammatory cytokines in infants with congenital cardiac defects. J Am Coll Cardiol. 2003;41:2266-74.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor necrosis factor alpha influences the inflammatory response after coronary surgery. Ann Thorac Surg. 2006;81:132-7.
- [CrossRef] [PubMed] [Google Scholar]
- Perioperative cytokine release during coronary artery bypass grafting in patients of different ages. Clin Exp Immunol. 1998;114:26-32.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor necrosis factor gene polymorphism is associated with enhanced systemic inflammatory response and increased cardiopulmonary morbidity after cardiac surgery. Anesth Analg. 2003;97:944-9.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative and postoperative endotoxemia in children with congenital heart disease. Chest. 2000;117:1706-12.
- [CrossRef] [PubMed] [Google Scholar]
- Myocardial inflammatory activation in children with congenital heart disease. Crit Care Med. 2002;30:827-32.
- [CrossRef] [PubMed] [Google Scholar]
- Transcriptomic analysis of patients with tetralogy of Fallot reveals the effect of chronic hypoxia on myocardial gene expression. J Thorac Cardiovasc Surg. 2010;140:337-45.e26.
- [CrossRef] [PubMed] [Google Scholar]
- Serum TNF-α levels in children with congenital heart disease undergoing cardiopulmonary bypass: A cohort study in China and a meta-analysis of the published literature. J Clin Lab Anal. 2017;31:e22112.
- [CrossRef] [PubMed] [Google Scholar]






