Translate this page into:
Malnutrition-Inflammation Liaison in Predicting AKI following OPCABG in Diabetics: Role of a Novel Monocyte/High-Density Lipoprotein × Albumin Ratio
Souvik Dey, MD Department of Cardiac Anaesthesia, Atal Bihari Vajpayee Institute of Medical Sciences (ABVIMS) and Dr. Ram Manohar Lohia Hospital Baba Kharak Singh Marg, New Delhi 110001 India souvik_23march@yahoo.in
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background Monocyte/high-density lipoprotein ratio (MHR) has been recently proposed as a parsimonious inflammatory marker. Akin to MHR, hypoalbuminemia (a malnutrition marker) has a considerable proinflammatory potential and confers an accentuated risk of postcardiac surgery complications, like acute kidney injury (AKI). The present study evaluated the AKI-predictive value of the preoperative monocyte/high-density lipoprotein × albumin ratio (MHAR) in diabetic patients undergoing off-pump coronary artery bypass grafting (OPCABG).
Methods The retrospective study conducted at our tertiary cardiac care center included 687 diabetic OPCABG patients. Forty-eight hours postoperative data was evaluated for the occurrence of AKI, as defined by the Acute Kidney Injury Network criteria. The perioperative attributes of the AKI and non-AKI groups were compared to evaluate the predictors of AKI, by employing a regression analysis.
Results A total of 109 patients (15.87%) developed AKI postoperatively. On univariate analysis: age, EuroSCORE II, preoperative congestive heart failure, systemic hypertension, serum albumin, MHR, MHAR, intraoperative packed red blood cell transfusion, postoperative low cardiac output syndrome, and vasoactive-inotropic score (VIS) predicted AKI. AKI subsequent to multivariate analysis, age (odds ratio [OR]: 1.029), EuroSCORE II (OR: 1.264), MHAR (OR: 1.403), and VIS (OR: 1.07) were the independent predictors (p-values: 0.020, < 0.001, 0.013, 0.002, respectively). The AKI predictive cutoffs of albumin, MHR, and MHAR were ≤ 2.95, ≥ 15.25, and ≥ 4.08 (area under the curve:sensitivity:specificity of 0.761:84.86%:89.62%; 0.754:93.12%:86.68%; 0.798:89.63%:88.34%, respectively). MHAR ≥ 4.08 was also associated with a higher incidence of postoperative atrial fibrillation and major adverse cardiac events.
Conclusion Preoperative elevated MHAR independently predicts AKI following OPCABG in diabetics, implying a malnutrition-inflammation liaison at heart of the matter.
Keywords
acute kidney injury
diabetes mellitus
malnutrition
monocyte/high-density lipoprotein ratio (MHR)
monocyte/high-density lipoprotein × albumin ratio (MHAR)
inflammation
off-pump coronary artery bypass grafting
Highlights
-
Monocyte/high-density lipoprotein ratio (MHR) has captivated attention as a parsimonious proinflammatory marker albeit remains underevaluated in cardiac surgery.
-
The recent proposition of MHR as a marker of diabetic nephropathy is also noteworthy.
-
Preoperative hypoalbuminemia (related to both poor nutritional status and proinflammatory predisposition) demonstrates an independent prognostic importance in cardiac surgical setting.
-
We retrospectively discovered a heightened risk of postoperative acute kidney injury (AKI) in off-pump coronary artery bypass grafting (OPCABG) diabetic patients with a preoperative monocyte/high-density lipoprotein × albumin ratio (MHAR) ≥ 4.08.
-
The former highlights that poor preoperative nutritional and proinflammatory profile potentially liaises to result in an accentuated postoperative cardiac-surgical morbidity.
Introduction
Metabolic inflammation (metaflammation) constitutes a topical research area, particularly in diabetic patients with an underlying coronary artery disease (CAD).1, 2, 3 This becomes all the more relevant amidst an ever-growing cohort of diabetic CAD.4 While the underlying baseline low-grade inflammation can be potentially exaggerated in the perioperative period,5, 6 the importance of preoperative malnutrition in modulating the postoperative cardiac surgical outcomes can also not be undermined.7
Appropriate to the context, hypoalbuminemia, conventionally envisaged as a preoperative marker of malnutrition, has an important proinflammatory potential as well.8 Albumin classifies as a negative acute phase reactant9 and hence, hypoalbuminemia can significantly contribute to an ongoing inflammatory process. Furthermore, an elevated monocyte/high-density lipoprotein ratio (MHR) has been recently described to be a parsimonious proinflammatory marker of prognostic importance in operative10, 11, 12 and nonoperative CAD settings.13, 14, 15, 16 The elucidation of coexistence of low serum albumin levels and high MHR in postcoronary artery bypass grafting (CABG) patients with saphenous vein graft disease by Akboga et al, is intriguing.17 The former provides subtle clues to the communion between poor nutritional status and proinflammatory predisposition.
With regards to the postoperative cardiac surgical complications, cardiac surgery associated-acute kidney injury (CSA-AKI) transpires with an accentuated frequency in diabetics and accounts for a considerable morbidity and mortality.18, 19 Alongside the recent proposition of MHR as a biomarker of diabetic nephropathy,20 hypoalbuminemia has also been described to be associated with an enhanced risk of CSA-AKI.21, 22 Therefore, the present study sought to retrospectively evaluate the AKI-predictive value of preoperative monocyte/high-density lipoprotein × albumin ratio (MHAR) in diabetic patients undergoing off-pump CABG (OPCABG). The secondary objectives were to compare the incidence of postoperative low cardiac output syndrome (LCOS), major adverse cardiac events (MACE), atrial fibrillation (AF), and sepsis, in the patients demonstrating preoperative MHAR values higher than the AKI-predictive cutoff and those having MHAR values less than the computed cutoff.
Materials and Methods
Following a formal approval from the Institutional Ethics Committee (No.601 (18/2020) IEC/ABVIMS/RMLH), the data of 813 diabetic patients, that is, patients who presented with fasting serum glucose > 126 mg/dL or on treatment with oral hypoglycemic medications/insulin, who had undergone elective OPCABG at our tertiary care referral center from January 2015 to December 2020, was retrospectively collected and evaluated. The patients who underwent emergency surgery, those with preoperative chronic kidney disease, and preoperative critically ill state (patients on mechanical ventilation, intra-aortic balloon pump [IABP], and/or inotropes) were excluded from the study. The patients who had systemic disorders potentially linked to hypoalbuminemia, such as hepatic dysfunction, active malignancy, certain endocrine disorders, for example, hypothyroidism, hyperthyroidism, etc., low hemoglobin (Hb) levels (≤ 10 g/dL), active infection, active or chronic autoimmune disease, lymphoproliferative disease, and patients on long-term treatment with steroids or chemotherapeutic drugs, were also excluded. The patients who required intraoperative cardiopulmonary bypass and/or postoperative IABP support, were excluded from the study. The lack of laboratory data within three preoperative days before was deemed as a study exclusion. After considering the loss to follow-up, the data of 687 patients was accumulated from the hospital archives and electronic database. The patient enrollment is illustrated as a flowchart in Fig. 1.

- The flow diagram depicting the patient enrolment methodology. AKI, acute kidney injury; CKD, chronic kidney disease; IABP, intra-aortic balloon pump; OPCABG, off-pump coronary artery bypass grafting.
Preoperative patient data including age, sex, body mass index, European System for Cardiac Operative Risk Evaluation (EuroSCORE II), prior myocardial infarction (MI), peripheral vascular disease, hypertension, chronic obstructive pulmonary disease, smoking history, preoperative congestive heart failure (CHF), preoperative drugs (β blockers, statins, angiotensin-converting enzyme inhibitors), left ventricle ejection fraction, and left main disease, history of coronary angiogram within 7 days preoperatively, etc. were noted. The laboratory parameters obtained were: Hb, total leucocyte count, absolute monocyte count (AMC), high-density lipoprotein (HDL), serum creatinine, aspartate transaminase, alanine transaminase, and serum albumin levels. The intraoperative and postoperative parameters namely duration of surgery, number of distal vessel anastomosis, LCOS, vasoactive-inotropic score (VIS), postoperative AF, postoperative sepsis, MACE following surgery, requirement of renal replacement therapy (RRT), postoperative duration of mechanical ventilation (DO-MV), length of intensive care unit stay (LOS-ICU), and length of hospital stay (LOS-H), were also documented.
MHR and MHAR were then calculated as per the following formulae:
• MHR = AMC/HDL
• MHAR = MHR/Albumin
The induction and maintenance of anesthesia, was as per a predefined institutional protocol. Volume-controlled ventilation was employed with oxygen and air in the ratio of 0.6 to result in an end tidal carbon dioxide concentration of 35 mm Hg along with 1.0 minimum alveolar concentration isoflurane. A Swan-Ganz catheter (Edwards Lifesciences, Irvine, California, United States) was inserted in the pulmonary artery (PA) via a 7Fr sheath placed in right internal jugular vein for monitoring PA pressures. The intraoperative bispectral index was maintained between 40 and 60.
After a midline sternotomy, left internal mammary artery and saphenous venous grafts were harvested. Upon administering heparin in a dose of 200 IU/kg of body weight and reaching an activated clotting time of > 300 seconds, the octopus evolution tissue stabilizer (Medtronic, Inc, Minneapolis, Minnesota, United States) was placed to stabilize the diseased coronary artery and inserting the intracoronary shunts. The distal anastomosis was executed with 6–0 or 7–0 prolene sutures, while proximal anastomosis was achieved using 5–0 prolene sutures. Once the coronary anastomosis concluded, protamine dose in a ratio of 1 mg/100 IU heparin was used to reverse the anticoagulation and all the patients were subsequently transferred to cardiac ICU for further management.
With respect to the glucose homeostasis, the glucose levels were evaluated at hourly intervals with arterial blood gas measurements. The perioperative blood glucose levels were regulated with an insulin infusion to the target glucose of 140 to 180 mg/dL.
Analysis of the 48 hours postoperative data was performed to determine the incidence of de novo AKI, defined by the Acute Kidney Injury Network (AKIN) criteria23 as:
-
AKIN stage 1: increased creatinine level > 50% or 0.3 mg/dL from baseline, or urine output < 0.5 mL/kg/h continuing more than 6 hours.
-
AKIN stage 2: increased creatinine level by > 100%, or urine output < 0.5 mL/kg/h over 12 hours.
-
AKIN stage 3: increased creatinine level by > 200% or serum creatinine level > 4 mg/dL with an acute increase of > 0.5 mg/dL, new-onset RRT, urine output < 0.3 mL/kg/h over 24 hours, or anuria over 12 hours.
The intraoperative and postoperative mean arterial pressure was maintained above 65 mm Hg24 with the assistance of vasopressors and inotropic support, as per the institutional regimen. The corresponding hemodynamic support was quantified as VIS25 wherein, VIS = Dopamine (µg/kg/min) + dobutamine (µg/kg/min) + milrinone (µg/kg/min) × 10 + epinephrine (µg/kg/min) × 100 + norepinephrine (µg/kg/min) × 100 + vasopressin (µg/kg/min) × 100 + vasopressin (µg/kg/min) × 1000.
Postoperative MACE was defined by the occurrence of any of the following: cardiac arrest, MI, and/or the need of repeat coronary revascularization.26
Statistical Analysis
The categorical variables of the AKI and non-AKI groups were converted to numerical values and percentages and analyzed using the chi-square test. The continuous variables were denoted as mean ± standard deviation and evaluated using the unpaired t-test. For assessing the correlation among continuous variables, Pearson's correlation analysis was applied. The nonparametric receiver operating characteristic (ROC) curve analysis was used to elucidate the accuracy of all variables in predicting AKI demonstrated by their respective area under the curve (AUC). The precise AKI-predictive cutoff was calculated as the cutoff mark with the maximum [(sensitivity + specificity)/2] ratio, at which there was the highest accuracy of postoperative AKI as an outcome. The sensitivity, specificity, and predictive values were then reported utilizing the created cutoff. The multivariate analysis was performed with binary logistic regression. The statistical software SPSS version 20 (IBM Corp, Armonk, New York, United States) was applied for the analysis. A confidence interval (CI) of 95% with 80% power of study, with a p-value of < 0.05 was considered as significant.
Results
The 687 OPCABG patients enrolled in the study comprised of 563 males (81.95%) and 124 females (18.05%). A total of 109 patients (15.87%) developed postoperative AKI. The demographic and perioperative characteristics of these patients, as categorized by the manifestation of postoperative AKI, are shown in Tables 1 and 2.
|
Parameter |
AKI group (n = 109) |
Non-AKI group |
p-Value |
|---|---|---|---|
|
Age (y) |
65.72 ± 8.84 |
62.6 ± 9.30 |
< 0.001 |
|
Female |
21 (19.27) |
103 (17.82) |
0.719 |
|
BMI (kg/m2) |
24.25 ± 2.33 |
23.98 ± 2.00 |
0.301 |
|
Smoking |
43 (39.45) |
198 (34.26) |
0.297 |
|
Family history |
50 (45.87) |
314 (54.33) |
0.105 |
|
Hypertension |
71 (65.14) |
304 (52.6) |
0.016 |
|
Hyperlipidemia |
62 (56.88) |
302 (52.25) |
0.374 |
|
Peripheral vascular disease |
12 (11.01) |
76 (13.15) |
0.540 |
|
COPD |
6 (5.5) |
24 (4.15) |
0.607 |
|
Preop CHF |
17 (15.6%) |
38 (6.57) |
< 0.001 |
|
Prior MI |
26 (23.85) |
167 (28.89) |
0.283 |
|
LVEF (%) |
48.05 ± 7.60 |
49.45 ± 7.18 |
0.083 |
|
EuroSCORE-II |
3.6 ± 1.6 |
3.0 ± 1.7 |
< 0.001 |
|
Left main disease |
37 (33.94) |
151 (26.12) |
0.093 |
|
Preoperative CAG |
22 (20.18) |
106 (18.33) |
0.651 |
|
ACE inhibitor use |
46 (42.2) |
232 (40.14) |
0.687 |
|
Statin use |
53 (48.62) |
303 (52.42) |
0.467 |
|
Beta blocker use |
62 (56.88) |
352 (60.9) |
0.432 |
|
Hb (g/dL) |
12.02 ± 1.26 |
12.18 ± 0.70 |
0.148 |
|
TLC (×103/mm3) |
8.24 ± 1.99 |
8.07 ± 1.83 |
0.818 |
|
ANC (cells/mm3) |
4137 ± 1306 |
4041 ± 911 |
0.242 |
|
ALC (cells/mm3) |
3252 ± 887 |
3364 ± 918 |
0.260 |
|
AMC (cells/mm3) |
543 ± 110 |
526 ± 109 |
0.073 |
|
HDL (mg/dL) |
38.06 ± 6.24 |
39.72 ± 8.54 |
0.259 |
|
MHR |
14.75 ± 4.28 |
13.8 ± 3.94 |
0.037 |
|
Serum creatinine (mg/dL) |
1.11 ± 0.59 |
0.99 ± 0.36 |
0.518 |
|
Serum albumin (g/dL) |
3.34 ± 0.65 |
3.53 ± 0.61 |
0.003 |
|
MHAR |
4.60 ± 1.61 |
4.08 ± 1.54 |
< 0.001 |
|
AST (IU/L) |
56.23 ± 14.59 |
57.59 ± 15.39 |
0.404 |
|
ALT (IU/L) |
55.64 ± 14.46 |
58.25 ± 15.69 |
0.235 |
Abbreviations: ACE, angiotensin-converting enzyme; AKI, acute kidney injury; ALC, absolute lymphocyte count; ALT, alanine aminotransferase; AMC, absolute monocyte count; ANC, absolute neutrophil count; AST, aspartate transaminase; BMI, body mass index; CAG, coronary angiogram; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; EuroSCORE-II, European System for Cardiac Operative Risk Evaluation II; Hb, hemoglobin; HDL, high-density lipoprotein; LVEF, left ventricular ejection fraction; MHAR, monocyte/high-density lipoprotein × albumin ratio; MHR, monocyte/high-density lipoprotein ratio; MI, myocardial infarction; TLC, total leucocyte count.
Note: Data are presented as a mean ± standard deviation or number (%). p-Values< 0.05 are italicized.
|
Parameter |
AKI group (n = 109) |
Non-AKI group (n = 578) |
p-Value |
|---|---|---|---|
|
Duration of surgery (min) |
253.93 ± 30.84 |
249.83 ± 26.51 |
0.199 |
|
No. of grafts |
2.89 ± 0.59 |
2.86 ± 0.51 |
0.274 |
|
PRBC transfused |
1.21 ± 0.77 |
0.95 ± 0.82 |
0.002 |
|
Mean CVP (mm Hg) |
10.40 ± 2.85 |
9.99 ± 1.91 |
0.101 |
|
Mean VIS |
14.72 ± 5.71 |
12.83 ± 4.72 |
< 0.001 |
|
LCOS |
7 (6.42) |
9 (1.56) |
0.007 |
|
Postoperative RRT |
9 (8.25) |
0 (0.00) |
< 0.001 |
|
Duration of MV (h) |
82.07 ± 48.12 |
71.19 ± 42.60 |
0.030 |
|
LOS ICU (d) |
4.67 ± 2.01 |
4.22 ± 1.78 |
0.030 |
|
LOS hospital (d) |
8.66 ± 1.99 |
8.06 ± 1.69 |
0.015 |
Abbreviations: AKI, acute kidney injury; CVP, central venous pressure; ICU, intensive care unit; LCOS, low cardiac output syndrome; LOS, length of stay; MV, mechanical ventilation; PRBC, packed red blood cell; RRT, renal replacement therapy; VIS, vasoactive-inotropic score.
Note: Data are presented as a mean ± standard deviation or number (%). p–Values < 0.05 are italicized.
The following variables were found to be significant predictors of postoperative AKI in the univariate analysis: advanced age, a higher logistic EuroSCORE II, elevated MHR, MHAR, and lower preoperative serum albumin concentrations. Patients with AKI were also more likely to have preexisting comorbidities such as hypertension and CHF. In addition, the packed red blood cell units transfused during surgery, postoperative LCOS, and mean VIS were also associated with the development of postoperative AKI (Table 3). The patients with AKI, demonstrated a higher requirement of RRT, DO-MV, LOS-ICU, and LOS-H, in contrast to those without AKI (Table 2). Preoperative MHAR was independently associated with postoperative AKI (multivariate logistic analysis; odds ratio [OR]: 1.403; 95% CI: 1.073–1.833; p-value: 0.013). Age (OR: 1.029; 95% CI: 1.005–1.054; p-value: 0.020), EuroSCORE II (OR: 1.264; 95% CI: 1.1–1.451; p-value: < 0.001), and VIS (OR: 1.074; 95% CI: 1.027–1.123; p-value: 0.002) also emerged as independent predictors of postoperative AKI, as outlined in Table 3.
|
Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
|
Parameter |
OR |
95% CI |
p-Value |
OR |
95% CI |
p-Value |
|
Age |
1.038 |
1.014–1.061 |
< 0.001 |
1.029 |
1.005–1.054 |
0.020 |
|
Hypertension |
1.594 |
1.388–1.910 |
0.016 |
1.297 |
0.806–2.087 |
0.295 |
|
Preop CHF |
1.381 |
1.206–1.703 |
< 0.001 |
1.351 |
0.745–2.197 |
0.074 |
|
EuroSCORE-II |
1.270 |
1.112–1.450 |
< 0.001 |
1.264 |
1.100–1.451 |
< 0.001 |
|
MHR |
1.058 |
1.007–1.112 |
0.037 |
1.171 |
0.963–1.428 |
0.120 |
|
Serum albumin |
0.619 |
0.448–0.854 |
0.003 |
0.351 |
0.129–0.951 |
0.085 |
|
MHAR |
1.493 |
1.153–1.932 |
< 0.001 |
1.403 |
1.073–1.833 |
0.013 |
|
PRBC transfused |
1.214 |
1.075–1.370 |
0.002 |
1.146 |
0.924–1.282 |
0.199 |
|
VIS |
1.083 |
1.037–1.130 |
< 0.001 |
1.074 |
1.027–1.123 |
0.002 |
|
LCOS |
1.809 |
1.344–2.837 |
0.007 |
1.772 |
0.762–2.703 |
0.091 |
Abbreviations: AKI, acute kidney injury; CHF, congestive heart failure; CI, confidence interval; EuroSCORE-II, European System for Cardiac Operative Risk Evaluation II; LCOS, low cardiac output syndrome; MHAR, monocyte/high-density lipoprotein × albumin ratio; MHR, monocyte/high-density lipoprotein ratio; OR, odds ratio; PRBC, packed red blood cell; VIS, vasoactive-inotropic score.
Note: p–Values < 0.05 are italicized.
Herein, albumin ≤ 2.95, MHR ≥ 15.25, and MHAR ≥ 4.08 (AUC; 95% CI; sensitivity; specificity: 0.761; 0.698–0.822; 84.86%; 89.62% [p-value < 0.001], 0.754; 0.694–0.814; 93.12%; 86.68% [p-value = 0.015], and 0.798; 0.74–0.856; 89.63%; 88.34% [p-value < 0.001], respectively) were the estimated AKI-predictive cutoffs based on the ROC curve analysis (Fig. 2). Interestingly, the study subset who had a preoperative MHAR > 4.08 (the computed AKI predictive cutoff) also demonstrated a higher incidence of postoperative AF and MACE (Table 4).
|
Parameter |
MHAR ≥ Cutoff |
MHAR < Cutoff |
p-Value |
|---|---|---|---|
|
MACE |
14 (2.04) |
6 (0.87) |
0.016 |
|
AF |
98 (14.26) |
52 (7.57) |
< 0.001 |
|
LCOS |
7 (1.02) |
9 (1.31) |
0.305 |
|
Sepsis |
5 (0.73) |
8 (1.16) |
0.079 |
Abbreviations: AF, atrial fibrillation; LCOS, low cardiac output syndrome; MACE, major adverse cardiac events; MHAR, monocyte/high-density lipoprotein × albumin ratio.
Note: Data are presented as number (%). p-Values < 0.05 are italicized.
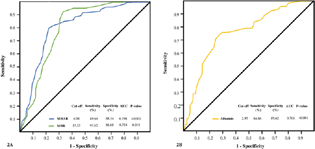
- (A) The AKI-predictive potential of MHR and MHAR assessed in context of AUC under ROC curve delineating the highest AUC of 0.798 for MHAR. The AKI-predictive cutoff values of the parameters have been presented in the lower part of the figure. (B) ROC curve for albumin in terms of AUC delineating the highest AUC of 0.761 for prediction of AKI. The predictive cutoff value of the albumin has been presented in the lower part of the figure. AKI, acute kidney injury; AUC, area under curve; MHAR, monocyte/high-density lipoprotein × albumin ratio; MHR, monocyte/high-density lipoprotein ratio; ROC, receiver operating characteristic.
Discussion
The demonstration of an independent AKI-predictive value of MHAR highlights that poor preoperative nutritional status potentially liaises with a prevailing proinflammatory milieu to result in an accentuated postoperative morbidity. As far as AKI (primary outcome under evaluation) is concerned, we discovered an incidence of 15.87% AKI in our diabetic subset undergoing OPCABG. The considerable variation in the reported incidence of 3.5 to 31% of CSA-AKI emanates as a result of the heterogeneity of the diagnostic criteria employed, surgical cohort under evaluation (off-pump or on-pump), design of the study including the exclusion criterion, and the procedure-specific predilection.19 Despite a homogeneous inclusion of OPCABG patients, the evaluation of an isolated diabetic subset could have attributed an incremental risk for postoperative AKI in our setting. Talking of the factors which independently predicted AKI in the index analysis, EuroSCORE II, in general implies an elevated cardiac surgical risk27 whereas a higher vasopressor-inotropic support (estimated as VIS), has also been linked to morbid postoperative outcomes.28 The other two independent AKI-predictive factors, including advanced age and MHAR in diabetic patients, herald a plausible constellation of inflammageing and metaflammation.2
In the context of a CSA-AKI-predictive value of MHAR outlined in the present study, the literature is replete with the prognostic importance of preoperative MHR10, 11, 12, 13, 14, 15, 16 and albumin.21, 22 While substantial research proposes a prognostic role of MHR in nonoperative settings (including cardiac patients),13, 14, 15, 16 there remains a dearth of studies featuring the role of the former in cardiac surgery. Herein, the findings of the research group led by Saskin et al deserve mention, which highlight the association of an elevated preoperative MHR with postoperative AF in a retrospective analysis of 662 patients undergoing on-pump CABG.10 They outline a MHR ≥ 0.0185 as the AF-predictive cutoff (AUC: 0.835; sensitivity: 84.6%; specificity: 81.0%) whereas a MHR ≥ 15.25 (AUC: 0.754; sensitivity: 93.12%; specificity: 86.68%) emerged as the AKI-predictive cutoff in our study. This is explained by the fact that Saskin et al consideration of monocyte cell lineages introduced a divisible factor of 1,000 to obtain the corresponding MHR values.10
Akin to the study by Saskin et al10 and another study by Tekkesin et al,11 we also discovered a higher incidence of postoperative AF in our patients having a preoperative MHAR values higher than the computed AKI-predictive cutoff. In addition, MACE transpired with a higher incidence in these patients which is in harmony with the Cetin et al13 description of an elevated risk of MACE in patients of acute coronary syndrome with an elevated MHR.
In another evaluation of 145 patients who underwent transcatheter aortic valve implantation by Karahan and Okuyan, the mortality remarkably increased from 4.1 to 76.1% owing to an elevation of MHR above the median value of 13.73.12 Alongside a 50% mortality risk accentuation for every 1 unit increase in MHR in the study, the ratio correlated positively with C-reactive protein, suggesting a proinflammatory predisposition at the cornerstone of an enhanced mortality.12 Albeit an underlying CAD being implicated for such an exorbitant mortality association of MHR by Karahan and Okuyan, Dolapoglu et al29 failed to discover any association between MHR and morbidity–mortality following CABG. Despite a degree of contradiction in the preliminary reports, the recent description of MHR as a biomarker of diabetic nephropathy by Karatas et al20 supports our finding of an AKI-predictive value of MHAR in diabetic cardiac surgical patients.
The prognostic importance of preoperative hypoalbuminemia can simultaneously not be underemphasized. Specific to literature on coronary revascularization, low albumin levels predict the risk of postoperative AKI, both in diabetics and nondiabetics.21, 22 Interestingly, the Akboga et al observation of low serum albumin levels in post-CABG patients (with saphenous vein graft disease) demonstrating higher MHR, pinpoints the possible links between the two independent markers of prognostic importance.17 At the same time, the depiction of an all-cause mortality predictive value of monocyte/albumin ratio in settings of percutaneous coronary interventions by Zhang et al, is equally noteworthy.30
Delving into the pathophysiology, the incremental value of MHAR over MHR and albumin possibly highlights a perilous liaison of malnutrition and inflammation. MHR derives its prognostic value from the combined proinflammatory potential of monocyte activation and the anti-inflammatory effects of HDL.31 This becomes all the more relevant in the light of literature to support an augmented targeted priming of monocytes to an inflammatory state in patients with lipid profile perturbations.32 While MHR can hence be equated with proinflammation, the dual relationship of hypoalbuminemia with malnutrition and inflammation,8 is expected to compound the postoperative outcomes furthermore. This is adequately highlighted in the anti-inflammatory, antioxidative stress and osmotic effects of albumin.8 The aforementioned explains the synergy behind the independent prognostic role of the novel MHAR highlighted in our multivariate analysis.
Strengths and Limitations
To the best of the authors' knowledge, the present study classifies as a maiden research endeavor of evaluating the AKI-predictive of a novel ratio, MHAR. Moreover, the study included a homogeneous cohort of diabetic patients who are peculiarly predisposed to AKI. The isolated evaluation of OPCABG patients additionally obviates the possible deleterious renal effects of extracorporeal circulatory conduct.33, 34 However, the study had a few limitations. First, the retrospective study design is prone to residual confounding.35 This is particularly pertinent in the context of CSA-AKI which is essentially multifactorial.33, 34 Second, the lack of availability of the renal oxygenation monitoring trends (near-infrared spectroscopy based) for all the included cases, is an additional limitation.36 Third, the inclusion of other markers of malnutrition, frailty, and/or inflammation could have enhanced the lucidity of the study findings.
Conclusion
Preoperative elevation of MHAR independently predicted postoperative AKI in the retrospective evaluation of our diabetic patients undergoing OPCABG. While the novel ratio concocts the nutritional and inflammatory predisposition and can potentially assist risk stratification and individualization37, 38 of targeted therapy, future prospective studies are warranted to validate the initial encouraging findings across diverse clinical settings.
Conflict of Interest
None declared.
References
- Metainflammation in diabetic coronary artery disease: emerging role of innate and adaptive immune responses. J Diabetes Res. 2016;2016:6264149.
- [Google Scholar]
- Inflammageing and metaflammation: the yin and yang of type 2 diabetes. Ageing Res Rev. 2018;41:1-17.
- [Google Scholar]
- Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177-185. (7640):
- [Google Scholar]
- Glycemic index, glycemic load, and cardiovascular disease and mortality. N Engl J Med. 2021;384(14):1312-1322.
- [Google Scholar]
- Endothelial glycocalyx and cardiac surgery: newer insights. J Cardiothorac Vasc Anesth. 2020;34(1):310-311.
- [Google Scholar]
- Systemic immune-inflammation index predicts poor outcome after elective off-pump CABG: a retrospective, single-center study. J Cardiothorac Vasc Anesth. 2021;35(8):2397-2404.
- [Google Scholar]
- Is malnutrition associated with postoperative complications after cardiac surgery? J Card Surg. 2019;34(10):908-912.
- [Google Scholar]
- Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432-437.
- [Google Scholar]
- Acute-phase proteins: as diagnostic tool. J Pharm Bioallied Sci. 2011;3(1):118-127.
- [Google Scholar]
- High preoperative monocyte count/high-density lipoprotein ratio is associated with postoperative atrial fibrillation and mortality in coronary artery bypass grafting. Interact Cardiovasc Thorac Surg. 2017;24(3):395-401.
- [Google Scholar]
- The use of monocyte to HDL ratio to predict postoperative atrial fibrillation after aortocoronary bypass graft surgery. North Clin Istanb. 2017;4(2):145-150.
- [Google Scholar]
- Monocyte-to-high density lipoprotein cholesterol ratio as a predictor of mortality in patients with transcatheter aortic valve replacement. Eur Rev Med Pharmacol Sci. 2021;25(16):5153-5162.
- [Google Scholar]
- Monocyte to HDL cholesterol ratio predicts coronary artery disease severity and future major cardiovascular adverse events in acute coronary syndrome. Heart Lung Circ. 2016;25(11):1077-1086.
- [Google Scholar]
- Monocyte-to-high density lipoprotein ratio (MHR) can predict the significance of angiographically intermediate coronary lesions. Int J Cardiovasc Acad. 2017;3:16-20.
- [Google Scholar]
- Relation of monocyte/high-density lipoprotein cholesterol ratio with coronary artery disease in type 2 diabetes mellitus. Clin Lab. 2018;64(6):901-906.
- [Google Scholar]
- Increased monocyte to high-density lipoprotein cholesterol ratio is associated with TIMI risk score in patients with ST-segment elevation myocardial infarction. Rev Port Cardiol (Engl Ed). 2018;37(3):217-223. (Engl Ed)
- [Google Scholar]
- Relationship between serum albumin level and monocyte-to-high-density lipoprotein cholesterol ratio with saphenous vein graft disease in coronary bypass. Thorac Cardiovasc Surg. 2017;65(4):315-321.
- [Google Scholar]
- Influence of diabetes mellitus on long-term survival in systematic off-pump coronary artery bypass surgery. Ann Thorac Surg. 2008;86(4):1181-1188.
- [Google Scholar]
- Acute kidney injury after cardiac surgery in patients without chronic kidney disease. Rev Bras Cir Cardiovasc. 2018;33(5):454-461.
- [Google Scholar]
- Monocyte to high-density lipoprotein cholesterol ratio in patients with diabetes mellitus and diabetic nephropathy. Biomarkers Med. 2018;12(9):953-959.
- [Google Scholar]
- Is hypoalbuminemia a predictor for acute kidney injury after coronary bypass grafting in diabetes mellitus patients? Rev Bras Cir Cardiovasc. 2019;34(5):565-571.
- [Google Scholar]
- Preoperative hypoalbuminemia is a major risk factor for acute kidney injury following off-pump coronary artery bypass surgery. Intensive Care Med. 2012;38(9):1478-1486.
- [Google Scholar]
- Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31.
- [Google Scholar]
- Hemodynamic monitoring in shock and implications for management. International Consensus Conference, Paris, France, 27-28 April 2006. Intensive Care Med. 2007;33(4):575-590.
- [Google Scholar]
- Vasoactive-inotropic score: evolution, clinical utility, and pitfalls. J Cardiothorac Vasc Anesth. 2021;35(10):3067-3077.
- [Google Scholar]
- Implication of major adverse postoperative events and myocardial injury on disability and survival: a planned subanalysis of the ENIGMA-II trial. Anesth Analg. 2018;127(5):1118-1126.
- [Google Scholar]
- Goal-directed therapy improves the outcome of high-risk cardiac patients undergoing off-pump coronary artery bypass. Ann Card Anaesth. 2017;20(1):83-89.
- [Google Scholar]
- Vasoplegic syndrome after cardiac surgery: better the devil you know! J Card Surg. 2019;34(12):1679-1680.
- [Google Scholar]
- Monocyte count to HDL-cholesterol level ratio on post-operative outcome after coronary bypass surgery. Tepecik Egit ve Arast HastDergisi. 2018;28:187-190.
- [Google Scholar]
- Monocyte-to-albumin ratio as a novel predictor of long-term adverse outcomes in patients after percutaneous coronary intervention. Biosci Rep. 2021;41(7):BSR20210154.
- [Google Scholar]
- Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045-2051.
- [Google Scholar]
- Monocyte inflammatory profile is specific for individuals and associated with altered blood lipid levels. Atherosclerosis. 2017;263:15-23.
- [Google Scholar]
- Nitric oxide: renoprotective in cardiac surgery! Rev Bras Cir Cardiovasc. 2020;35(4):602-603.
- [Google Scholar]
- Effect of goal-directed therapy on post-operative neutrophil gelatinase-associated lipocalin profile in patients undergoing on-pump coronary artery surgery. Indian J Thorac Cardiovasc Surg. 2019;35(3):445-452.
- [Google Scholar]
- Renal near-infrared spectroscopy for assessment of renal oxygenation in adults undergoing cardiac surgery: a method validation study. J Cardiothorac Vasc Anesth. 2020;34(12):3300-3305.
- [Google Scholar]
- Implications of practice variability: comment. Anesthesiology. 2020;133(4):943-944.
- [Google Scholar]
- Precision cardiac anesthesia: welcome aboard! J Cardiothorac Vasc Anesth. 2020;34(9):2551-2552.
- [Google Scholar]







