Translate this page into:
Echocardiography for Left Ventricular Assist Device Patients
Poonam Malhotra Kapoor, MD, DNB, MNAMS, FISCU (Hony), FIACTA (Hony), FTEE (Hony) Department of Cardiac Anaesthesia and Critical Care, All India Institute of Medical Sciences New Delhi 110029 India drpoonamaiims@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Heart transplantation is the only cure for patients with end-stage heart failure; the shortage of donors has led to a high mortality rate among these patients. A left ventricular assist device (LVAD) is a device to provide mechanical circulatory support for patients unresponsive to heart failure therapy. Echocardiography should be considered an indispensable tool in the evaluation of patients with an LVAD. In fact, as outlined in this review, it provides useful and readily available information that could be crucial for the patient's survival. In the preoperative assessment, it is important to detect through echocardiography conditions and parameters that could hint the development of a postoperative complication, in order to treat them before LVAD implant or to consider the patient ineligible to this advanced treatment.
Keywords
echocardiography
left ventricular assist device
left ventricular function
Introduction
Heart transplantation is the only cure for patients with end-stage heart failure (HF); the shortage of donors has led to a high mortality rate among these patients. A left ventricular assist device (LVAD) is a device to provide mechanical circulatory support for patients unresponsive to HF therapy.
Patients receive LVAD therapy based on clinical need for following - LVAD used as a:
-
Bridge to transplant
-
Bridge to candidacy
-
Destination therapy
-
Bridge to recovery.
Types of Ventricular Assist Device
The VADs are electromechanical pumps, usually positioned in parallel with the circulation, which comprise the following components:
-
An inflow cannula that brings the blood from one of the heart chambers to the device.
-
A pump for propulsion of blood.
-
An outflow cannula that returns the blood to the patient.
-
A controller which receives and processes information from the pump and returns information for pump operation.1, 2
The blood propulsion mechanism also ranges from traditional roller pumps (e.g., Stockert) to pulsatile pumps (HeartMate I, Thoratec) and up to recent axial-flow devices (e.g., HeartMate II, Jarvik 2000) and centrifugal pumps (Biomedicus and Tandem Heart) devices.
The HeartMate II is a continuous axial-flow pump that consists of a propeller in a pump (Fig. 1).

- Left ventricular assist device and its components.
The parts of the HeartMate II (Fig. 2) are positioned in succession—rotor pump, inflow cannula, outflow cannula, and a single drive line that exits percutaneously towards the electronic controller—to act as the left ventricle.
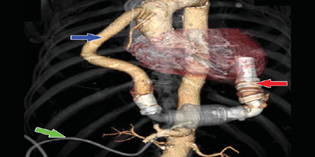
- HeartMate II (Thoratec Corp., Pleasanton, California, United States, green arrow—drive line, red arrow—inflow cannula, blue arrow—outflow cannula.
The inflow cannula is inserted into the apex of the LV.
The outflow cannula is anastomosed to the right anterior aspect of the ascending aorta.
The LVAD pump is placed within the peritoneal cavity.
The spinning of the rotor draws blood from the inflow cannula through cardiac diastole and systole into the ascending aorta (Fig. 1).
Use of echocardiography for LVAD
The echocardiography is integral in four areas in a LVAD placement:
-
Preoperative assessment of patients.
-
Guidance during VAD insertion.
-
Detection of complications.
-
Monitoring the cardiac recovery.
Pre-VAD Insertion and ECHO
Two main purposes of echo are:
-
To determine the suitability of LVAD implantation for a patient with HF
-
To identify abnormalities that could predict complication postoperatively especially the assessment of the ventricles.
Left Ventricle Assessment (LV)
LV function pre-LVAD insertion will show depressed function with a dilated or normal size ventricle, depending on the cause of heart failure. The left ventricular ejection fraction for LVAD insertion is typically 25 to 30%. Significant diastolic failure is also present and diagnosed with pulsed wave (PW) Doppler of the transmitral inflow combined with PW Doppler of the pulmonary veins or with tissue Doppler. The presence of a restrictive LV diastolic function implies increased LV and left atrial (LA) pressures, when severe, it supports the indication. Severe LV dysfunction is a high risk for apical thrombus formation. Apical thrombus is often located near the inflow cannula insertion site. A preoperative echo of the LV for the presence of thrombus is essential. Due to imaging orientation and scan planes, transesophageal echocardiogram (TEE) may not detect LV apical thrombi and transthoracic echocardiogram can have a better diagnostic yield. Intravenous (IV) echocardiographic contrast injection can help increase the sensitivity of detecting LV apical thrombus (Fig. 3).
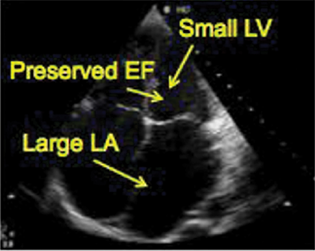
- Left ventricle echocardiography.. EF, ejection fraction; LV, left ventricular.
Right Ventricular Assessment
Right ventricular (RV) dysfunction is a well-recognized and serious condition that can occur in LVAD recipients, as the output of the native RV determines the preload of the LVAD. A decrease in RV function will cause a reduction in the LVAD output.3
The RV dysfunction after cardiopulmonary bypass (CPB) remains difficult to predict in the individual patient, because of its multifactorial nature, but preoperative identification of risk factors for RV dysfunction helps to select patients who would benefit from biventricular assistance and improve clinical outcomes by preventing period of low output from RV failure. A LVAD can have a beneficial effect on the RV by reducing afterload or a detrimental effect by increasing preload to an already compromised RV and by impairing RV contractility through leftward septal shift during LVAD support. A preoperatively dilated RV with increased RV preload and afterload predisposes to RV dysfunction after LVAD implantation (Fig. 4a).
- Right ventricle echocardiography.
Views to Assess RV-Two-Dimensional Images
-
The midesophageal four-chamber (Fig. 4a)
-
Transgastric short- and long-axis views (Fig. 4b)
-
ME RV inflow–outflow (Fig. 4C).
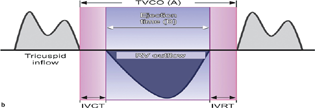
- Tei index. IVRT, isovolumetric relaxation time; RV, right ventricular; TVCO, tricuspid valve co.
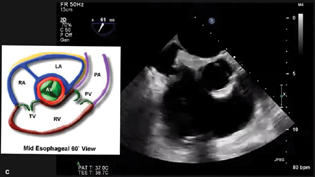
- Midesophageal RV Inflow Outflow View.
What to look for?
-
Semiquantitative assessment of RV function and dilation
-
RV base (tricuspid valve [TV] annulus) to apex motion and free-wall motion
-
Quantitative analysis of RV function and dilation
-
The maximum derivative of the RV pressure (dP/dt max) have been used to quantify systolic function.
Global RV fractional area change: The global RV fractional area change is most often used, in patients undergoing LVAD implantation. It is usually between 20 and 30%.
The regional fractional area change: Patients with a RV fractional area change less than 20% may suffer RV failure after LVAD insertion. Detection of a moderately depressed RV is also important, as it will guide towards inodilator therapy for decreasing pulmonary hypertension, increasing RV contractility and strict volume management to minimize volume overload to the right heart after separation from CPB. The TV inflow velocity profile has been used as a measure of RV diastolic function.
A more quantitative evaluation of RV myocardial performance is the Tei index (Fig. 4b). The Tei index is reproducible, independent of ventricular geometry, and not significantly impacted by heart rate or blood pressure. Ventricular loading conditions do not appear to affect the index. The calculation for the Tei index is done using the TV annular velocity.
Tei index = (a − b)/b.
A result ≤0.4 is normal.
Limitations of the Tei index include pseudonormalization and the fact that it cannot be measured in patients with arrythmias.
TAPSE Right for RV Function of LVAD
TAPSE is a method to measure the distance of systolic excursion of the RV annular segment along its longitudinal plane from apical four-chamber view.
It assumes that the displacement of the basal and adjacent segments in the apical four-chamber view is representative of the function of the entire RV, an assumption that is not valid in many disease states.
-
A TAPSE cutoff value of 17 mm yields high specificity to distinguish abnormal from normal systolic function.
-
Functional tricuspid regurgitation (TR) is a common finding in patients with HF. Severe postoperative TR contributes to RV dysfunction and leads to a low output state.
-
The mechanism of TR is extremely important to define and moderate or greater may require surgical correction at the time of LVAD implantation.
-
Significant TV annular dilation represents a risk factor for post-LVAD implant TR.
-
Absence of TR does not imply that the TV orifice is free from abnormality.
-
All these variables reinforce the importance of a meticulous examination of RV size and function and TV pre-LVAD implantation.
-
Assessment of the ascending aorta (Figure 8)
– The outflow cannula of an LVAD device is usually attached to the ascending aorta in as end to side anastomosis.
– The two main abnormalities that impact on LVAD insertion are aneurysmal dilatation of the ascending aorta and atherosclerotic disease.
– If there is significant dilatation of the ascending aorta (>45 mm) detected at the time of LVAD insertion, the ascending aorta is usually replaced with a Dacron graft and the LVAD cannula attached in an end to side manner to the graft.3
-
Assessment of native cardiac valves
– Dysfunction of all four cardiac valves can have an impact of management of patients at the time of VAD insertion.
– The presence of hemodynamically significant mitral stenosis at the time of LVAD insertion usually results in surgical correction at the same time, via either a commissurotomy of mitral valve replacement, using a tissue mitral valve.
– Mitral regurgitation is commonly associated with end-stage heart failure. With adequate unloading of the ventricle post LVAD insertion, there is often improvement in the degree of mitral regurgitation.
– Uncorrected aortic regurgitation has a negative impact on forward flow provided by an LVAD due to regurgitation of VAD flow back into the left ventricular cavity. Moderate and greater levels of severity of aortic regurgitation should be corrected at the time of VAD insertion.
– Aortic stenosis is usually not of such clinical significance compared to aortic regurgitation. However, in continuous flow devices, depending upon the configuration, aortic stenosis may limit forward flow and may need surgical replacement at the time of VAD insertion.
– TR is a relatively common condition in patients being assessed for mechanical support. This is typically due to right heart failure secondary to chronic elevation of the pulmonary pressures due to left heart failure.
Assessment of prosthetic cardiac valves
– Patients with prosthetic valves in situ who are being considered for VAD represent a complex group in terms of their management.
– The key issue here is the risk of prosthetic valve thrombosis due to low flow states across the valve in the setting of a mechanically supported circulation. This may occur with both biological and mechanical valves, though the risk is likely to be higher with mechanical valves. If a mechanical valve is in situ at the time of VAD insertion, remove the mechanical valve and insert a biological one instead
Right-to-left shunting (atrial and ventricular)
Pulmonic insufficiency (RVAD)
Ventricular scar, particularly at cannulation site
Guidance during VAD Insertion
The key components in this evaluation are assessment:
-
Satisfactory cannula anatomic positioning within the cardiac chambers
-
Determination of adequate flows within the cannulae using Doppler imaging
-
Cannula flow pattern: Inflow and outflow (before and after chest closure): color, continuous wave, and PW
-
Adequate chamber decompression
-
The presence of air within the circuit.
The apical inflow cannula can be confirmed with four-chamber (evaluate deviation toward the interventricular septum [IVS]) (Fig. 5) and two-chamber views (to evaluate anteroposterior direction) (Figs. 6 and 7).
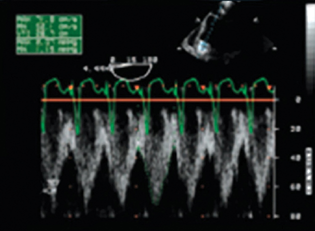
- Inflow cannula Doppler profile (normal) cannula obstruction.
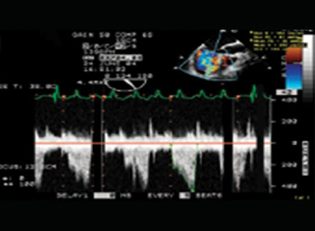
- Doppler flow turbulence—inflow.
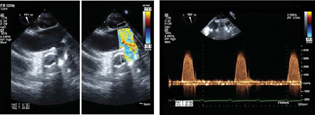
- (A and B) Left ventricular assist device outflow anastomosis in ascending aorta (ME Asc aorta long axis color Doppler).
– Patent foramen ovale (PFO) search should always be performed before and after CPB for implantation of a VAD.
– Agitated saline or bubble study and color Doppler TEE are highly sensitive and specific methods for the diagnosis of a PFO and for estimating the degree of shunt flow.
– In the prebypass, increased LA pressure causes rightward deviation of interatrial septum. Because of this, PFO with color flow may show a left-to-right shunt.
But a bubble study may not show a PFO due to the difficulty in producing a transient reversal of the left-to-right pressure gradient in the presence of left heart failure.
– In the case of biventricular failure, increased right atrial (RA) and LA pressures reduce the interatrial pressure gradient, and PFO detection by both agitated saline and color Doppler fails.
After insertion of a LVAD, there is LV unloading with decrease in the LA pressure. This in association with increased right heart pressures may uncover a PFO and favor a paradoxical embolism leading to development of severe hypoxemia in the presence of pulmonary hypertension. Significant right-to-left shunting can be visualized with TEE, clinically as severe hypoxemia upon activation of the LVAD.
– TEE assessment for PFO after VAD insertion can be started early, while weaning from CPB. Early detection is important, because the presence of a PFO requires return to CPB for closure.
– Traumatic atrial septal defects also produce profound hypoxemia in the setting of increased right-to-left atrial pressure gradient with LVAD support. The degree of shunting across an interatrial septal defect may be aggravated by chest closure.
– Ventricular septal defects, for example, postinfarct, can be a cause of shunting before and after VAD placement.
-
Right ventricular assessment after LVAD implantation:
– Achievement of LV unloading should be balanced with RV function since augmenting pump speeds can increase venous return and worsen RV performance. Echocardiographic methods used to assess RV function after LVAD implantation are similar as preoperative evaluations that are RV morphology, function, and severity of TR.
– Hemodynamic data should include estimates of RV systolic pressure (peak TR velocity) and mean RA pressure (inferior vena cava measurements)
Factors increasing TR are the following:
-
Leftward shift of the IVS
-
Decreased RV function post-LVAD implant
-
LVAD function increasing RV preload to an already dysfunctional RV.
The factor blunting TR is as follows:
-
TR may be blunted or absent when the right atrial (RA) pressure is very high.
The following is a beneficial effect whereby TR is reduced:
-
Unloading of the LV decreases TR and pulmonary artery pressure.
The leftward IVS shift is more pronounced in LVAD patients who are hypovolemic, have reduced RV function, or significant TR pre-LVAD implant.
Patients with elevated LVAD flow will demonstrate an IVS shift culminating in a spiral “suction event.” This finding mandates decreasing the pump speed with a resultant reduction of TR and improved RV function.
– Tamponade following chest closure
– Ascending and descending aorta: dissection
– Aortic insufficiency
– Inflow and outflow valve dysfunction
– Left ventricular unloading.
Device specific:
-
Pulsatile devices:
– Continuous closure of the aortic valve
– Pulsatile flow velocities in device cannulas
-
Axial flow devices:
– Intermittent opening of the aortic valve
– Pulsatile flow velocities in device cannulas superimposed on continuous velocity (Fig. 8)
– Left ventricular unloading, right ventricular function and TR according to flow settings.
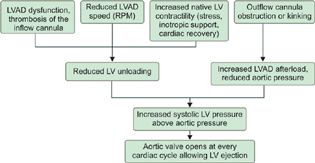
- Left ventricular assist device dysfunction or thrombosis. LVAD, left ventricular assist device.
Hemodynamic Instability in Early Postoperative Days
-
Hypovolemia: Should be considered when the RV and LV cavities are small.
-
Acute RV dysfunction.
-
Cardiac tamponade.
-
Pulmonary embolism.
-
Suction cascade: Most typical expression of acute RV dysfunction.
-
Echo findings: Dilated and hypocontractile RV, significant functional TR, small left ventricle, and an intermittent inflow cannula obstruction by the collapsed LV wall.
-
LVAD dysfunction or thrombosis (Fig. 8) should be suspected when clinically (cardiogenic shock, low LVAD output), echocardiographic (rightward deviation of the interventricular and interatrial septum, significant functional mitral regurgitation, aortic valve opening every cardiac cycle and the diastolic aortic pressure to exceed the LV diastolic pressure, resulting in a reverse flow from the ascending aorta through the outflow and inflow cannulas), and laboratory clues (intravascular hemolysis) are detected.6
Conclusion
TEE plays a major role in evaluating perioperative structure and function related both to the patient's heart and large vessels and to the implanted device. This evaluation is key for anesthetic and surgical planning and success of VAD implantation. Echocardiography should be considered an indispensable tool in the evaluation of patients with an LVAD.7, 8, 9, 10 In fact, as outlined in this review, it provides useful and readily available information that could be crucial for the patient's survival. In the preoperative assessment, it is important to detect through echocardiography conditions and parameters that could hint the development of a postoperative complication, in order to treat them before LVAD implant or to consider the patient ineligible to this advanced treatment. During the implant, echocardiography provides information on whether the device is correctly placed as well as on the response of RV to the activation of LVAD. During the patient's follow-up, it is possible to define the correct functioning of the device as well as possible malfunctioning. Postop rules out thrombosis and LVAD malfunction.
Conflict of Interest
None declared.
References
- Echocardiographic Evaluation of Ventricular Assist Devices. Intechopen Limited Publisher, London United Kingdom; 2011. David Platts.
- [Google Scholar]
- Perioperative echocardiographic examination for ventricular assist device implantation. Anesth Analg. 2007;105(3):583-601.
- [Google Scholar]
- The ABCs of left ventricular assist device echocardiography: a systematic approach. Eur Heart J Cardiovasc Imaging. 2012;13(11):885-899. Nov;
- [Google Scholar]
- The practical role of echocardiography in selection, implantation, and management of patients requiring LVAD therapy. Curr Cardiol Rep. 2014;16(4):468.
- [Google Scholar]
- Echocardiography in the Management of Patients with Left Ventricular Assist Devices: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015;28(8):853-909.
- [Google Scholar]
- Echocardiography for left ventricular assist device implantation and evaluation: an indispensable tool. Heart Fail Rev. 2022;27(3):891-902.
- [Google Scholar]
- Echocardiographic assessment for ventricular assist device placement. J Thorac Dis. 2015;7(12):2139-2150.
- [Google Scholar]
- Echocardiographic Variables After Left Ventricular Assist Device Implantation Associated With Adverse Outcome. Circulation: Cardiovascular Imaging. 2011;4(6):648-661.
- [Google Scholar]
- Echocardiography and Continuous-Flow Left Ventricular Assist Devices: Evidence and Limitations. JACC: Heart Failure. 2015;3(7):554-564.
- [Google Scholar]
- The Role of Echocardiography and Other Imaging Modalities in Patients With Left Ventricular Assist Devices. JACC: Cardiovascular Imaging. 2010;3(10):1049-1064.
- [Google Scholar]






