Translate this page into:
Risk of Developing Acute Kidney Injury on the VA ECLS Circuit in Patients with Acute Decompensated Heart Failure
Shams Reaz, DO Internal Medicine, University of Michigan-West 5900 Byron Center, Wyoming, Grand Rapids, MI United States shams.reaz@metrogr.org
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aims Venoarterial extracorporeal life support (VA ECLS) is a life-saving treatment with a high risk of mortality. Appropriate patient selection is critical for optimal patient outcomes. Acute kidney injury (AKI) is a common risk among VA ECLS patients, and more information is needed to understand how AKI affects the mortality risk of these patients. To do this, we examined acute decompensated heart failure (ADHF) patients supported by VA ECLS and compared their risk of developing AKI to a background population. VA ECLS has become an increasingly important tool to bridge or recover patients with severe ADHF as the primary indication of VA ECLS.
Methods and Results All VA ECLS patients from a single center were included. ADHF patients supported by VA ECLS were compared with the remaining VA ECLS cohort. CATEGORICAL comparisons were made between groups using chi-squared and Fisher's exact tests. A survival analysis was conducted to determine freedom from AKI between the two groups. Predictor variables were tested by multiple logistic regression. Of the 255 patients included in this study, 110 had ADHF as their primary indication for VA ECLS and 145 patients had other VA ECLS indications. The survival analysis showed that patients with ADHF had a decreased risk of developing AKI on the VA ECLS circuit. Multiple logistic regression revealed no predictors in AKI development between groups and no difference in 30-day mortality was observed.
Conclusion Patients supported by VA ECLS are at high risk of mortality and complications. This research demonstrated that medically complex ADHF patients had less chance of developing AKI when compared with other patients supported by VA ECLS. Future research is needed to investigate potential protective mechanisms of VA ECLS support.
Keywords
extracorporeal membrane oxygenation
acute kidney injury
acute decompensated heart failure
Introduction
Venoarterial extracorporeal life support (VA ECLS) use has expanded exponentially over the last decade for both circulatory and pulmonary support in the critical care setting. Despite technical progress, mortality on VA ECLS has been relatively stable around 50 to 60%.1, 2, 3 Patients supported by VA ECLS are at risk of several complications, with acute kidney injury (AKI) being among the most common.4 Previous research has reported that as many as 60 to 85% of VA ECLS patients require continuous renal replacement therapy (CRRT) for AKI.4, 5, 6, 7 Development of AKI is associated with high mortality in VA ECLS patients, with up to a 78% increased rate of mortality.1, 2, 3, 4, 5, 6, 8
Patients with decompensated heart failure who require VA ECLS support have been shown to have a higher risk of mortality within 4 days of VA ECLS initiation when compared with other cardiac patients on VA ECLS support.2 Further, there is a known cardiorenal interaction that is prevalent in patients with heart failure,9, 10 with heart failure patients having an increased risk of renal dysfunction.9, 11 Therefore, patients with heart failure who develop AKI while on VA ECLS support may have poor prognosis.
There is currently a lack of clarity in understanding how impaired renal function affects prognosis in heart failure patients. Research investigating outcomes in patients who develop AKI while on the VA ECLS circuit is necessary so that clinicians may better assess and select appropriate patients for this support. It is thought that patients may develop AKI while supported by VA ECLS due to the changes in hemodynamics and perfusion taking place in the body; however, the precise mechanisms remain unclear.6 Heart failure patients are already at high risk due to comorbidities and poor prognosis,12 and VA ECLS support should be used only in cases where it may improve survival. For these reasons, we decided to assess development of AKI and mortality risk in all VA ECLS patients at our institution. The study aimed to investigate whether the growing population of acute decompensated heart failure (ADHF) patients on VA ECLS has any difference in risk of developing AKI on VA ECLS when compared with the rest of the VA ECLS cohort.
Methods
Patients
The VA ECLS registry was approved by the Spectrum Health Institutional Review Board and received a waiver of consent. This was a retrospective cohort study based on patients requiring VA ECLS support at a single quaternary medical center, from the start of the VA ECLS program in June 2010 through February 2019. All VA ECLS patients, with the exception as below, were eligible. Patients were grouped based on indication for VA ECLS: ADHF patients versus the rest (e.g., post-cardiotomy, acute myocardial infarction, cardiac arrest not requiring extracorporeal resuscitation, and others). Patients with post heart transplantation primary graft dysfunction or myocarditis and patients with enhanced cardiopulmonary resuscitation were excluded from the study: the first two groups because their extraordinary good prognosis and the latter because their extraordinary poor prognosis. Patient data were collected from the medical record by both manual abstraction and electronically from the data warehouse. Data was stored in our local VA ECLS REDCap registry.13
Patients with AKI were identified based on KDIGO stage 1 criteria.14 The definition includes patients with a serum creatinine increase of 1.5 or more, a serum creatinine of greater than 0.3 mg/dL (26.5 μmol/L), or a urinary output of less than 0.5 mL/kg/h during a 6-hour block. This criterion was used to determine the timing of AKI development.
Statistics
Normally distributed continuous variables were compared by independent t-test and dichotomous variables were compared with Fisher's exact test. Data was presented as mean ± standard deviation. A survival analysis was performed with freedom from development of AKI on the circuit as the primary outcome. The patients with ADHF as primary diagnosis for VA ECLS were compared with the rest of the VA ECLS patients and displayed in a Kaplan–Meier curve. Between-group differences were compared using a log-rank test.
Multiple logistic regression was performed to predict the interdependency of ADHF with other clinical variables predictive of AKI (age, body mass index [BMI], alanine transaminase [ALT], modification of diet in renal disease estimated glomerular filtration rate [(MDRD eGFR], arterial pH, lactate, sodium, mean pulmonary artery pressure [PAP], and diastolic PAP), with development of AKI as the outcome. p-Values less than 0.05 were considered significant. Statistical analysis was performed using R statistical package.15
Results
Two-hundred fifty-five VA ECLS patients were eligible and included in this study. Of those, 110 had ADHF as their primary indication for VA ECLS, and there were 145 other VA ECLS patients in the comparison group. A list of modifiable and nonmodifiable factors was compared between the two groups in the single-center institution as reported in Table 1. First, MDRD eGFR was noted to be 50.9 mL/min/m2 in ADHF group compared with 57.58 mL/min/m2 in the remaining VA ECLS patients that was statistically significant (p = 0.016) in addition to difference in serum sodium level (138.6 mmol/L vs. 140.69 mmol/L, respectively; p = 0.002). This data demonstrates that ADHF patients come with a lower incidence of AKI on VA ECLS than the background VA ECLS population. Second, ADHF VA ECLS patients had significantly higher alanine transferase (ALT) and mean PAP than the rest of the VA ECLS patients (503.6 IU/L vs. 173.63 IU/L, p < 0.001 and 31.47 mm Hg vs. 28.2 mm Hg, p = 0.016, respectively: Table 1). However, the sequential organ failure assessment score was significantly worse in the primary ADHF population (10.7) than the background VA ECLS population (9.71, p = 0.005: Table 1) that is consistent with the overall prognosis of VA ECLS dependent ADHF patients nationwide.
|
Variable |
ADHF (n = 110) |
Other VA ECMO (n = 145) |
p-Value |
|---|---|---|---|
|
Mean ± SD |
Mean ± SD |
||
|
Age, yr |
55.86 ± 14.63 |
58.24 ± 13.75 |
0.301 |
|
Height, cm |
172.95 ± 10.24 |
172.39 ± 10.95 |
0.681 |
|
Weight, kg |
93.8 ± 24.27 |
90.16 ± 22.16 |
0.29 |
|
BMI, kg/m2 |
31.26 ± 7.44 |
30.22 ± 6.47 |
0.298 |
|
ALT, IU/L |
503.6 ± 1301.2 |
176.63 ± 655.88 |
<0.001 |
|
Bilirubin total, mg/dL |
1.04 ± 0.93 |
1.1 ± 0.84 |
0.472 |
|
pH arterial, mm Hg |
7.27 ± 0.18 |
7.34 ± 0.17 |
<0.001 |
|
Lactate, mmol/L |
6.01 ± 4.21 |
4.95 ± 3.56 |
0.055 |
|
MDRD eGFR, mL/min/1.73m2 |
50.9 ± 23.69 |
57.58 ± 24.85 |
0.016 |
|
Sodium, mmol/L |
138.6 ± 5.42 |
140.69 ± 5.98 |
0.002 |
|
Potassium, mmol/L |
4.38 ± 0.87 |
4.27 ± 0.59 |
0.666 |
|
CVP (RAP), mm Hg |
13.84 ± 7.97 |
13.42 ± 5.75 |
0.427 |
|
MAP arterial, mm Hg |
78.66 ± 50.18 |
71.98 ± 29.86 |
0.894 |
|
Core temperature, °C |
36.43 ± 1.14 |
36.29 ± 1.48 |
0.999 |
|
SVR |
1322.81 ± 922.99 |
1388.19 ± 941.17 |
0.574 |
|
Inotrope score |
20.74 ± 15.45 |
18.66 ± 21.3 |
0.171 |
|
SOFA score |
10.71 ± 3.05 |
9.71 ± 2.86 |
0.005 |
|
Perfusion |
57.5 ± 37.64 |
59.31 ± 18.89 |
0.121 |
|
Arterial systolic, mm Hg |
103.26 ± 35.89 |
102.59 ± 24.14 |
0.607 |
|
Arterial diastolic, mm Hg |
64.98 ± 33.43 |
62.98 ± 16.96 |
0.97 |
|
PAP mean, mm Hg |
31.47 ± 13.98 |
28.2 ± 11.29 |
0.016 |
|
PAP diastolic, mm Hg |
26.26 ± 11.58 |
23.26 ± 8.6 |
0.089 |
Abbreviations: ADHF, acute decompensated heart failure; ALT, alanine transaminase; BMI, body mass index; CVP, central venous pressure; MAP, mean arterial pressure; MDRD eGFR, modification of diet in renal disease estimated glomerular filtration rate; PAP, pulmonary artery pressure; RAP, right atrial pressure; SD, standard deviation; SOFA, sequential organ failure assessment; SVR, systemic vascular resistance; VA ECMO, venoarterial extracorporeal membrane oxygenation.
The survival analysis demonstrated that ADHF patients have a significantly lower overall risk in developing AKI during their first week on the circuit when compared with the background population (log-rank p = 0.005; Fig. 1). No difference in 30-day mortality was seen (Fig. 2). Further, a list of statistically significant variables ADHF, eGFR, PAP, lactate, sodium, ALT, and nonmodifiable variables age and BMI were identified as possible predictors for the development of AKI. The results of the multiple logistic regression showed no difference in variables as predictors of AKI, other than ADHF (Table 2). Therefore, ADHF in VA ECLS support has less risk of developing AKI compared with non-ADHF patients in agreement with survival analysis.

- Survival analysis of acute decompensated heart failure (ADHF) venoarterial extracorporeal membrane oxygenation (VA ECMO) patients compared with the remaining VA ECMO patients with acute kidney injury (AKI) as the outcome. ECLS, extracorporeal life support.
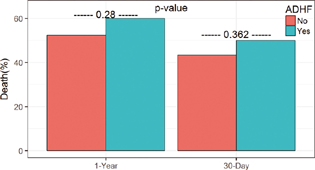
- One-year and 30-day mortality between acute decompensated heart failure (ADHF) venoarterial extracorporeal life support (VA ECLS) patients and the remaining VA ECLS patients.
|
Variable |
Estimate |
SE |
z-Value |
p-Value |
OR (95% CI) |
|---|---|---|---|---|---|
|
ADHF |
−0.939 |
0.434 |
−2.162 |
0.031 |
0.39 (0.17–0.92) |
|
Age (yr) |
−0.009 |
0.016 |
−0.591 |
0.554 |
0.99 (0.96–1.02) |
|
BMI (kg/m2) |
−0.05 |
0.035 |
−1.427 |
0.154 |
0.95 (0.89–1.02) |
|
ALT 10 unit (IU/L) |
−0.004 |
0.003 |
−1.276 |
0.202 |
1 (0.99–1) |
|
MDRD eGFR (mL/min/1.73m2) |
−0.013 |
0.009 |
−1.457 |
0.145 |
0.99 (0.97–1.01) |
|
Arterial pH 0.2 unit |
−0.416 |
0.294 |
−1.415 |
0.157 |
0.66 (0.37–1.17) |
|
Lactate (mmol/L) |
0.122 |
0.067 |
1.812 |
0.07 |
1.13 (0.99–1.29) |
|
Sodium (mmol/L) |
−0.075 |
0.171 |
−0.442 |
0.659 |
0.93 (0.66–1.3) |
|
PAP mean (mm Hg) |
−0.009 |
0.023 |
−0.397 |
0.692 |
0.99 (0.95–1.04) |
|
PAP diastolic (mm Hg) |
0.002 |
0.029 |
0.063 |
0.95 |
1 (0.95–1.06) |
Abbreviations: ADHF, acute decompensated heart failure; AKI, acute kidney injury; ALT, alanine transferase; BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; MDRD, modified diet renal disease; PAP, pulmonary artery pressure; OR, odds ratio; SE, standard error.
Discussion
The present study demonstrated that ADHF VA ECLS patients had a lower incidence of AKI when compared with other patients requiring VA ECLS support. Further, we observed no difference in 30-day mortality between the groups of VA ECLS patients. A previous study by Luo et al also observed a lower incidence of AKI in VA ECLS patients with ADHF when compared with VA ECLS patients requiring support for other indications.7 However, that study found an increased risk of in-hospital mortality among ADHF patients supported by VA ECLS. Our analysis of a patient cohort from nearly a decade later may reflect improvements in VA ECLS technology and survival over the years.
The results from both studies indicate that the patients with ADHF are at a lower risk of developing AKI than other VA ECLS patients, and the use of VA ECLS should not be discouraged in this patient population. While there is still a lack of understanding of the mechanisms involved in the development of AKI in VA ECLS patients, alternative theories propose that the use of VA ECLS may indeed provide a protective mechanism against AKI in some patients.16 One potential mechanism is improved renal perfusion in acutely ill patients. There is no evidence at this time to confirm whether this occurs in ADHF patients, but this could be a clinically significant area of future research.
Understanding AKI incidence in critically ill ADHF patients is of utmost importance since heart failure is a public health problem in the United States. The prevalence of heart failure has been increasing in recent years, from 5.7 million Americans between the years of 2009 to 2012, to 6.5 million Americans between the years of 2013 and 2016.17 The prevalence is expected to continue to rise, due to the aging population in the United States.17, 18 The current study indicated that ADHF patients are not at an increased risk of AKI, which is important evidence when it comes to treatment and decision-making of critically ill ADHF patients. All additional risks need to be considered in such complex patients, and these findings may help inform the medical community of improvements that can be made in the selection process.
The overall mortality rates of patients supported by VA ECLS are still an added problem for this high-risk population. Previous research has suggested that management of AKI may worsen clinical outcomes in heart failure patients.19 However, the present findings suggest that timely administration of VA ECLS support may yield favorable results in patients with ADHF requiring advanced support. In fact, in severe ADHF with AKI VA ECLS could be encouraged in contrast to the opposite.
This study was limited by the retrospective nature of the research. Due to the critical nature of this patient population, randomized clinical trials are not a possibility. This study examined the experience in an VA ECLS program at a single center over the course of 9 years. The protocols for patient management inevitably evolved over that time period; however, our findings align with much of the previous published research on adult VA ECLS patients.
In summary, while VA ECLS is a life-saving treatment for patients that may have not survived otherwise, it does not come without risks of morbidity and complication. It has been speculated whether there is a “friend or foe” relationship between kidney function and VA ECLS support.16 However, of patients supported by VA ECLS, ADHF patients were at decreased risk of developing AKI compared with patients without ADHF, with no difference in 30-day mortality between groups. Further research investigating protective mechanisms of VA ECLS support on kidney function in ADHF patients is warranted.
Conflict of Interest
None declared.
Funding Helen Devos Foundation, Spectrum Health Heart Institute, Van Andel Research Center.
References
- Prognosis of patients on extracorporeal membrane oxygenation: the impact of acute kidney injury on mortality. Ann Thorac Surg. 2011;91(1):137-142.
- [Google Scholar]
- Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: survival at five years. J Thorac Cardiovasc Surg. 2001;122(1):92-102.
- [Google Scholar]
- Risk factors for acute kidney injury and in-hospital mortality in patients receiving extracorporeal membrane oxygenation. PLoS One. 2015;10(10):e0140674.
- [Google Scholar]
- Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97(2):610-616.
- [Google Scholar]
- Renal function and survival in 200 patients undergoing ECMO therapy. Nephrol Dial Transplant. 2013;28(1):86-90.
- [Google Scholar]
- Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol. 2012;7(8):1328-1336.
- [Google Scholar]
- Extracorporeal membrane oxygenation for treatment of cardiac failure in adult patients. Interact Cardiovasc Thorac Surg. 2009;9(2):296-300.
- [Google Scholar]
- Acute kidney injury in adults receiving extracorporeal membrane oxygenation. J Formos Med Assoc. 2014;113(11):778-785.
- [Google Scholar]
- Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109(8):1004-1009.
- [Google Scholar]
- Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: a systematic review. Crit Care. 2014;18(6):675.
- [Google Scholar]
- Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102(2):203-210.
- [Google Scholar]
- Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27(1):65-75.
- [Google Scholar]
- Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
- [Google Scholar]
- A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27(12):4263-4272.
- [Google Scholar]
- R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria;
- [Publisher] [Google Scholar]
- The complex relationship of extracorporeal membrane oxygenation and acute kidney injury: causation or association? BioMed Res Int. 2016;2016:1094296.
- [Google Scholar]
- Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528.
- [Google Scholar]
- Who has advanced heart failure?: definition and epidemiology. Congest Heart Fail. 2011;17(4):160-168.
- [Google Scholar]
- Treatment patterns of patients with acute heart failure who develop acute kidney injury. ESC Heart Fail
- [Google Scholar]






