Translate this page into:
Perfusion Strategies for Bivalirudin Anticoagulation: AIIMS Protocol
Suruchi Hasija, MD, DM Department of Cardiac Anaesthesia and Critical Care, 7th Floor, Cardiothoracic Centre, All India Institute of Medical Sciences New Delhi 110029 India suruchi_hasija@hotmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Anticoagulation strategies for cardiac surgery are witnessing a change with the identification of serious limitations of heparin, including development of resistance in 3 to 13% of patients undergoing cardiac surgery and heparin-induced thrombocytopenia/thrombosis syndrome in 1 to 5.5% of patients. Heparin alternatives have a potential role in these scenarios. Bivalirudin, a reversible direct thrombin inhibitor, has an onset time of 2 to 4 minutes and half-life of 25 minutes, is eliminated mainly by a proteolytic mechanism, does not require antithrombin III for effect, and is nonimmunogenic. The considerations for extracorporeal circulation are peculiar with its use, and this article outlines the aspects of initiating, maintaining, and terminating cardiopulmonary bypass and extracorporeal membrane oxygenation with bivalirudin as the anticoagulant.
Keywords
anticoagulation
bivalirudin perfusion strategies
Introduction
Anticoagulation strategies for cardiac surgery are witnessing a change with the identification of serious limitations of heparin, including development of resistance in 3 to 13% of patients undergoing cardiac surgery and heparin-induced thrombocytopenia/thrombosis syndrome in 1 to 5.5% of patients.1 Heparin alternatives have a potential role in these scenarios. Bivalirudin, a reversible direct thrombin inhibitor, has an onset time of 2 to 4 minutes and half-life of 25 minutes, is eliminated mainly by a proteolytic mechanism, does not require antithrombin III for effect, is nonimmunogenic, and does not have an antidote. The considerations for extracorporeal circulation are peculiar with its use, but there are no standardized protocols. This article outlines the protocol followed at the authors' institution, highlighting the technical aspects of initiating, maintaining, and terminating cardiopulmonary bypass (CPB) and extracorporeal membrane oxygenation (ECMO) with bivalirudin as the anticoagulant.
Conduct of CPB with Bivalirudin as Anticoagulant
Pre-CPB
-
Heparin-coated oxygenators are not used. Instead, heparin-free oxygenators are used.
-
Heparin-coated tubings are not used. Instead, polyvinyl chloride tubings are used.
-
Bivalirudin is added while priming the CPB circuit, instead of heparin.
-
Blood is added to the prime just before CPB is commenced to ensure that bivalirudin levels are not depleted on exposure to blood.
-
Activated clotting time (ACT) sample is drawn 5 minutes after administering bivalirudin.
-
ACT of 400 s or 2.5 times baseline is ensured before cannulation and commencing CPB (Table 1).
|
Children younger than 1 year6 |
|||
|---|---|---|---|
|
Flush solutions, mg/mL |
0.1 |
0.1 |
0.1 |
|
Prime, mg |
50 |
50 |
25 |
|
Pre-CPB |
1 mg/kg followed by 2.5 mg/kg/h |
1.7 mg/kg followed by 3 mg/kg/h |
1 mg/kg followed by 2.2 mg/kg/h |
|
CPBa |
2.5 mg/kg/h |
3.0 mg/kg/h |
2.2 mg/kg/h |
|
ECMO |
Loading dose 0.1–1.0 mg/kg Maintenance dose 0.3–0.5 mg/kg/h |
||
Abbreviations: CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation.
CPB
-
It is of paramount importance to avoid stagnation in all components of the CPB circuit. Stasis of blood rapidly depletes the local concentration of bivalirudin and triggers the coagulation process.3, 4
-
ACT is monitored religiously every 30 minutes to ensure value above 400 s.
-
To avoid chamber and chest cavity stasis, cardiotomy suckers are frequently used.6
-
The potential areas where blood can stagnate are the shunt lines, cardioplegia circuit, and hemofilter. The shunt lines containing blood, for example, from oxygenator or arterial filter to reservoir are ideal sites for clot formation if not in use for long periods. Therefore, these are flushed at regular intervals of 15 minutes to avoid stagnation.4, 5
-
The choice of cardioplegia solution and circuit can be modified depending upon the surgical procedure. Use of sanguineous cardioplegia is a good option for surgeries that be completed in a shorter clamp time, whereas asanguineous cardioplegia covers for longer procedures.
-
While using sanguineous cardioplegia, there are chances of clot formation as the circuit remains idle for a period varying between 20 and 60 minutes. The cardioplegia pump/circuit is potentially at risk for clot formation in the time period between the cardioplegia delivery schedule (20 minutes for St. Thomas' solution and 60 minutes for del Nido solution).
-
The other option is to use asanguineous cardioplegia such as Bretschneider's (Custodiol) solution wherein the risk of clot formation is averted altogether. Crystalloid cardioplegia solutions give a safe cardiac arrest period of 90 minutes, which is usually sufficient for most cardiac surgeries. In case there is prolongation of arrest period, supplemental doses can be administered using a pressure bag and sparing the cardioplegia circuit.
-
Hemofilter is an important and integral part of CPB circuit, which not only removes extra volume but also reduces the inflammatory mediators. During the periods of CPB when hemofiltration is not being performed, clot formation can be avoided by allowing blood to continuously flow through the hemofilter by clamping the effluent port/filtrate outlet line (Fig. 1a).5
-
Bivalirudin (molecular weight: 2,180 Da) can pass through hemofilter membrane (pore size: 60,000–65,000 Da). It is eliminated to the extent of 45 to 69% and its half-life decreases by 20%.7 Therefore, dose escalation may be needed when hemofiltration is being performed.

- When hemofiltration is not being performed on CPB (a) or ECMO (b), simply clamping the effluent port allows continuous blood flow through the hemofilter and avoids stagnation.
Post-CPB
-
After weaning off CPB and stabilizing the patient hemodynamically, the circuit is kept on circulation by opening the recirculation line and connecting the arterial–venous loop.
-
Bivalirudin infusion is continued in the circuit till the patient is shifted and stable in the intensive care unit. This is to ensure that the circuit remains clot-free in case the need for a subsequent CPB run arises.
-
The volume in the venous reservoir may have to be built up adequately. To this, another 50-mg bolus of anticoagulant is added and the solution is circulated through the entire circuit, keeping all purge lines/shunt lines open.
Conduct of ECMO with Bivalirudin as Anticoagulant
-
It is of paramount importance to avoid stagnation in all components of the ECMO circuit. Stasis of blood rapidly depletes the local concentration of bivalirudin and can initiate the coagulation process (Fig. 2a,b).
-
Use of heparin-coated ECMO circuit is avoided.
-
As ECMO is a closed circuit, proper cannulation is essential to avoid airlock/air embolism. The arterial and venous cannulas with inbuilt Luer-lock ports are used.
-
A loading dose is unnecessary unless it is in the subtherapeutic range and/or in the context of suspected thrombosis (Table 2).8
-
Target ACT is 180 to 220 s and target activated partial thromboplastin time is 50 to 80 s.8
-
The use of three-way stopcocks is minimized as they are potential areas of initiation of clot formation. All the three ways are flushed at regular intervals or exchanged if required.
-
The shunt line clamp is released regularly.
-
Stagnation is avoided during hemofiltration by keeping blood flowing through it continuously (Fig. 1b). The ACT is checked periodically if hemofiltration is in use. Thrombosis in the ECMO circuit/membrane can lead to increase in circuit pressure/resistance causing rupture of the membrane.
-
Stagnation of blood is also avoided in the cardiac chambers if there is lack of ejection/contractility.9
-
A shunt line with one-way valve between arterial cannula and venous cannula is placed to keep the circuit in circulation for trial off or standby conditions (Fig. 3).
-
ECMO flow is increased whenever anticoagulation is reduced to minimize the risk of thrombus formation.
-
The pump flow rate is never reduced below the recommended flow rates for the given centrifugal pump.
-
A standby ECMO circuit is always made available.

- Blood in the backplate area of the centrifugal blood pump impairing magnetic coupling (a); clot formation along the bearing column (arrow, b)
|
Advantages |
|
• Safe and efficacious |
|
• Predictable response |
|
• Not associated with: |
|
– Resistance |
|
– Immunogenicity |
|
• Less platelet dysfunction |
|
• Inhibits free and clot-bound thrombin |
|
• Can be monitored with ACT plus |
|
Disadvantages |
|
• Mandates increased vigilance |
|
– Avoid stasis in all components of the CPB circuit |
|
– Looking out for clot formation |
|
– Frequent anticoagulation monitoring |
|
• Requires dose adjustment |
|
– Renal impairment |
|
– Hemofiltration |
|
• No reversal agent, extra time expended on surgical hemostasis |
|
• Cost |
Abbreviations: ACT, activated clotting time; CPB, cardiopulmonary bypass.
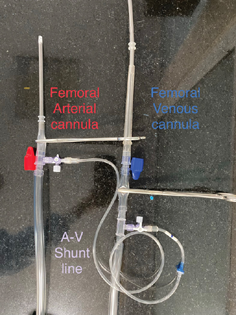
- A shunt line connection between arterial and venous lines of the standby ECMO circuit keeping most of the circuit in circulation.
Conflict of Interest
None declared.
References
- Kaplan's Cardiac Anesthesia: For Cardiac and Noncardiac Surgery. (7th ed.). Philadelphia, PA: Elsevier; 2017.
- [Google Scholar]
- A comparison of bivalirudin to heparin with protamine reversal in patients undergoing cardiac surgery with cardiopulmonary bypass: the EVOLUTION-ON study. J Thorac Cardiovasc Surg. 2006;131(3):533-539.
- [Google Scholar]
- Role of bivalirudin for anticoagulation in adult perioperative cardiothoracic practice. J Cardiothorac Vasc Anesth. 2020;34(8):2207-2214.
- [Google Scholar]
- The use of bivalirudin in pediatric cardiac surgery and in the interventional cardiology suite. J Cardiothorac Vasc Anesth. 2020;34(8):2215-2223.
- [Google Scholar]
- Randomized controlled trial of heparin versus bivalirudin anticoagulation in acyanotic children undergoing open heart surgery. J Cardiothorac Vasc Anesth. 2018;32(6):2633-2640.
- [Google Scholar]
- Bivalirudin anticoagulation in neonates and infants undergoing cardiac surgery. J Cardiothorac Vasc Anesth 2022
- [CrossRef] [Google Scholar]
- An assessment of different filter systems for extracorporeal elimination of bivalirudin: an in vitro study. Anesth Analg. 2003;96(5):1316-1319.
- [Google Scholar]
- Bivalirudin for alternative anticoagulation in extracorporeal membrane oxygenation: a systematic review. J Intensive Care Med. 2017;32(5):312-319.
- [Google Scholar]
- Anticoagulation with direct thrombin inhibitors during extracorporeal membrane oxygenation. World J Crit Care Med. 2019;8(6):87-98.
- [Google Scholar]






