Translate this page into:
Optimizing Red Blood Cell Mass, the First Pillar of PBM – First Things First
*Corresponding author: Nilmini Wijesuriya, Department of Anaesthesia, College of Anaesthesiologists and Intensivists of Sri Lanka, Rajagiriya, Sri Lanka. nilm314@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Wijesuriya N. Optimizing Red Blood Cell Mass, the First Pillar of PBM – First Things First. J Card Crit Care TSS. 2024;8:11-5. doi: 10.25259/JCCC_24S1_NW
Abstract
Patient blood management involves a three pillar approach to optimizing. The first pillar was optimizing the red cell mass. In doing so, a goal-oriented patient care approach is needed to obtain improved clinical outcomes. All of this requires the application of published evidence and utilizing best clinical practice.
Keywords
Patient blood management
Preoperative anemia
Iron deficiency
Transfusion
Iron therapy
INTRODUCTION
The World Health Organization (WHO) issued a policy brief on “Urgent need to implement patient blood management (PBM)” in 2020 with the aim of addressing a serious global health issue that has not received adequate attention.[1] PBM is a concept where patient safety is of prime importance and includes three pillars to detect and manage iron deficiency, anemia, minimization of blood loss and coagulopathy, and optimizing the patient-specific physiological tolerance to anemia. These pillars are common to both surgical and non-surgical patients. PBM is proposed by the National Blood Transfusion Committee in the UK and many other international societies and organizations.[2]
Detection of anemia and iron deficiency with the aim of finding the underlying cause and treatment with appropriate pharmacological agents and nutritional supplements is a central part of the first pillar. This review article aims to update the concept of PBM with emphasis on the first pillar of PBM, which is optimizing red cell mass.
DEFINITION
In 1968, the WHO defined anemia as hemoglobin (Hb) <130 g/L in adult males, 120 g/L in non-pregnant adult females, and <110 g/L for pregnant females. This definition is now more than 55 years old.[3]
Muñoz et al., have established an international consensus statement recommending a Hb of <130 g/dL be used as the definition for both sexes.[4] The Center for Perioperative Care (CPOC), which is a multidisciplinary initiative led by the Royal College of Anesthetists to improve perioperative care, published guidance in September 2022 addressing perioperative anemia, known to be associated with poorer patient outcomes across all surgical specialties.[5] The rationale for recommending a Hb <130 g/L for both sexes is the growing evidence that although a Hb of <130 g/L is commoner in women, it is suboptimal in the perioperative period. Having a lower body weight and a circulating blood volume but the same physiology of bleeding exposes a woman to a higher chance of reaching a set transfusion threshold earlier. This risk is increased if her baseline Hb is lower.[6]
The CPOC guideline includes both WHO and International consensus definitions. An intrinsic transfusion risk should be the main factor in determining which definition of anemia for women to adopt. Surgery-specific targets seem to be a rational approach.
PREVALENCE OF ANEMIA
It is not common knowledge that 27% of the world’s population is anemic. It affects 1.95–2.36 billion people globally. About 40% of pregnant women worldwide are also found to be anemic. About 14–64% of patients with chronic kidney disease are anemic. Those having malignancies have a prevalence of anemia between 26% and 53%. The elderly population is at a higher risk of anemia, and 10% of people over 65 years and 25% of people over 85 years are known to be anemic. Mild-to-moderate anemia is more prevalent in surgical patients than in the general population, and the incidence is from 30% to 80% in different studies.[7] Hospital-acquired anemia is a less recognized issue. More than one-third of patients who are not anemic on admission can develop anemia during admission.[8]
IMPACT OF ANEMIA ON SURGICAL OUTCOMES
It is estimated that more than 100 million surgeries are done on anemic patients every year globally. Perioperative anemia is an independent risk factor for increased mortality, prolonged hospital and intensive care stay, and postoperative complications such as poor wound healing and slow postoperative recovery. A meta-analysis of over 900,000 patients has found a significantly increased risk of acute kidney injury, infections, stroke in cardiac surgery, red blood cell transfusion, and perioperative mortality in patients with anemia.[9] The traditionally accepted treatment for anemia is to transfuse blood. Hazards of blood and blood product transfusions, such as circulatory overload, transfusion reactions, immunomodulatory effects, and infective complications, are well known.[10] Allogenic blood transfusion is itself an independent major predictor of postoperative morbidity and mortality. Transfusion of even a single unit of blood is shown to significantly increase 30-day mortality, risk of death, pneumonia, and sepsis.[11]
Oxygen delivery is compromised when Hb is below 60 g/L and tissue hypoxia and organ dysfunction become apparent. Effects of perioperative anemia are significant in older adults and patients with cardiac and pulmonary disease that compounds surgical stress. Therefore, perioperative anemia should be considered as an important modifiable factor, not just a simple abnormal value.
A locally applicable pathway to detect and optimize anemia early should be available at the time of the decision to operate by the surgical team. A multidisciplinary approach to managing these patients should be adopted to reduce modifiable risks associated with anemia in the perioperative period.
DETECTION AND MANAGEMENT OF ANEMIA IN THE PERIOPERATIVE PERIOD
Routine screening for anemia should be done for all patients at risk of blood loss of more than 500 mL or more than 10% of blood volume at the earliest opportunity to allow enough time for treatment. Checking a full blood count should be done as initial screening at least 4–6 weeks before surgery. A thorough history and examination are important to narrow down the differential diagnosis and to plan further blood tests or referrals to confirm the diagnosis. The decision to delay surgery to optimize Hb should be decided by senior surgical and anesthetic teams, taking the benefits and risks into consideration for the individual patient. If surgery is urgent, whatever opportunity is available before operation should still be utilized to investigate and initiate treatment.[12]
IRON DEFICIENCY ANEMIA
The most common treatable cause of preoperative anemia is iron deficiency, which accounts for up to 75% of preoperative anemia incidence. Causes of anemia can be classified into anemia related to iron metabolism and anemia not related to iron metabolism. Anemia related to iron deficiency can be categorized into absolute and functional iron deficiency, and they are mostly treatable.
Absolute iron deficiency in surgical patients can be due to increased loss (chronic bleeding from the gastrointestinal tract, menorrhagia, and genitourinary losses), the increased requirement (pregnancy, use of erythropoietin stimulating agents), or limited supply (iron-poor diet, poor absorption).
Functional iron deficiency represents the second most common cause of anemia, which is due to increased levels of hepcidin inhibiting iron absorption from the gut and release from macrophages of the reticular endothelial system. Therefore, total body iron is not reduced but sequestered in stores.[13]
Anemia not related to iron deficiency – can be due to multiple causes, and the mechanism may be reduced production of red cells due to nutritional deficiencies (Vitamin B12 and folate) and bone marrow pathologies. Inherited or acquired hemolytic disorders can cause increased destruction of red cells. Anemia could be the presentation of other uncommon hematological disorders such as thalassemia, myelodysplasia, and hematological malignancies where further hematological investigations are warranted.
DIAGNOSTIC WORKUP
A full blood count with low mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and MCH concentration indicates hypochromic microcytic anemia suggestive of iron deficiency anemia. However, similar findings can be seen in thalassemia, which is prevalent in Sri Lanka and iron deficiency can present with normal red cell indices, making these parameters not to be diagnostic.[14]
The other useful markers of body iron content are reticulocyte count, percentage of hypochromic cells (if more than 5%, suggestive of iron deficiency), and mean reticulocyte Hb content <29 pg. These changes are not specific to iron deficiency. Blood film examination can be a useful tool in detecting iron deficiency. Classical features observed in iron deficiency are hypochromic microcytic red blood cells with pencil-shaped cells, elliptocytes, and target cells. It can detect other conditions, such as myelodysplasia, thalassemia, and hemoglobinopathies.
Serum ferritin levels have 92% sensitivity and 98% specificity, and a serum level <30 μg/L is considered diagnostic of iron deficiency with or without anemia. Transferrin saturation (TSAT) is also recommended as an initial test in diagnosing iron deficiency anemia, where a level <20% is indicative of insufficient iron supply for erythropoiesis. According to the international consensus statement on the perioperative management of anemia and iron deficiency, the presence of inflammation (C-reactive protein >5 mg/dL), TSAT <20%, and ferritin <100 μg/L strongly suggest iron deficiency.[4] TSAT <20% and ferritin >100 μg/L usually indicate functional anemia as a result of iron sequestration.
Once iron deficiency anemia is diagnosed, the underlying cause should be identified. A common cause is chronic bleeding, and this necessitates referral to relevant specialists, such as gastroenterology, to exclude lesions and hematology for further differential diagnosis. Basic investigations such as inflammatory markers and renal and liver function tests are useful when evaluating anemia.
Other pathology, such as Vitamin B12/folate deficiency, liver pathology, thyroid disorders, and bone marrow pathology should be suspected in the presence of a high MCV due to macrocytosis.
MANAGEMENT OF PERIOPERATIVE ANEMIA
Default therapy for perioperative anemia is blood transfusion, which is one of the most common procedures performed during hospitalizations. Anemia and iron deficiency, blood loss and bleeding, and transfusion form a triad of independent risk factors for adverse outcomes. Therefore, transfusions given for iron deficiency may indicate inappropriate use of blood. Surgical blood loss stimulates increased erythropoiesis in the presence of adequate preoperative iron stores. Surgical patients with moderate to severe iron deficiency anemia should receive preoperative oral iron therapy. Early detection and diagnosis of anemia is important as oral iron takes up to 3 months to increase Hb significantly. It also needs good patient compliance. Oral iron therapy can cause side effects such as nausea and gastric discomfort.
Paying attention to all three pillars of PBM is necessary to manage perioperative anemia successfully. These strategies include not only the detection and management of anemia and iron deficiency but also minimizing blood loss, optimizing coagulation, and enhancing the patient-specific physiological tolerance to anemia.
Intravenous iron should be considered for patients with severe anemia who have poor tolerance to oral iron or lack of effectiveness, and surgery is scheduled within <4 weeks. The cost of iv iron roughly equals one unit of blood minus the hazards of transfusion. It can be given as a single dose, and improvement of functional status can be seen within four weeks, which may last for 24–52 weeks. Local guidelines and pathways should be in place with facilities to monitor and manage any complications, which are usually rare. The response to intravenous iron should be assessed by repeating a full blood count to decide whether the patient can proceed with surgery or whether further evaluation and treatment are necessary. Preoperative intravenous iron was not found to be superior to placebo to reduce transfusion needs or death in the perioperative period when given to anemic patients 10–42 days before elective major abdominal surgery. However, it was found to increase postoperative Hb levels and reduce readmission to hospital for surgical complications.[15]
A randomized open-label trial comparing intravenous iron and erythropoiesis-stimulating agent (ESA) versus oral iron to treat preoperative anemia in cardiac surgery found that a single dose of iv iron and ESA decreased the proportion of patients who received blood transfusion due to a greater increase in Hb when compared with oral iron.[16]
Intravenous iron can cause iron overload, worsen liver and kidney dysfunction, and hypertensive disorders. Caution should be exercised in previous allergies to iron or other pre-existing allergic conditions and in patients with ongoing bacterial infections. Intravenous iron should not be given to patients who received iv iron within the last seven days and in anemia not due to iron deficiency.
The following algorithm [Figure 1] modified from the international consensus statement on perioperative management of anemia and iron deficiency can be used for quick assessment of the cause of anemia and further workup.[4]
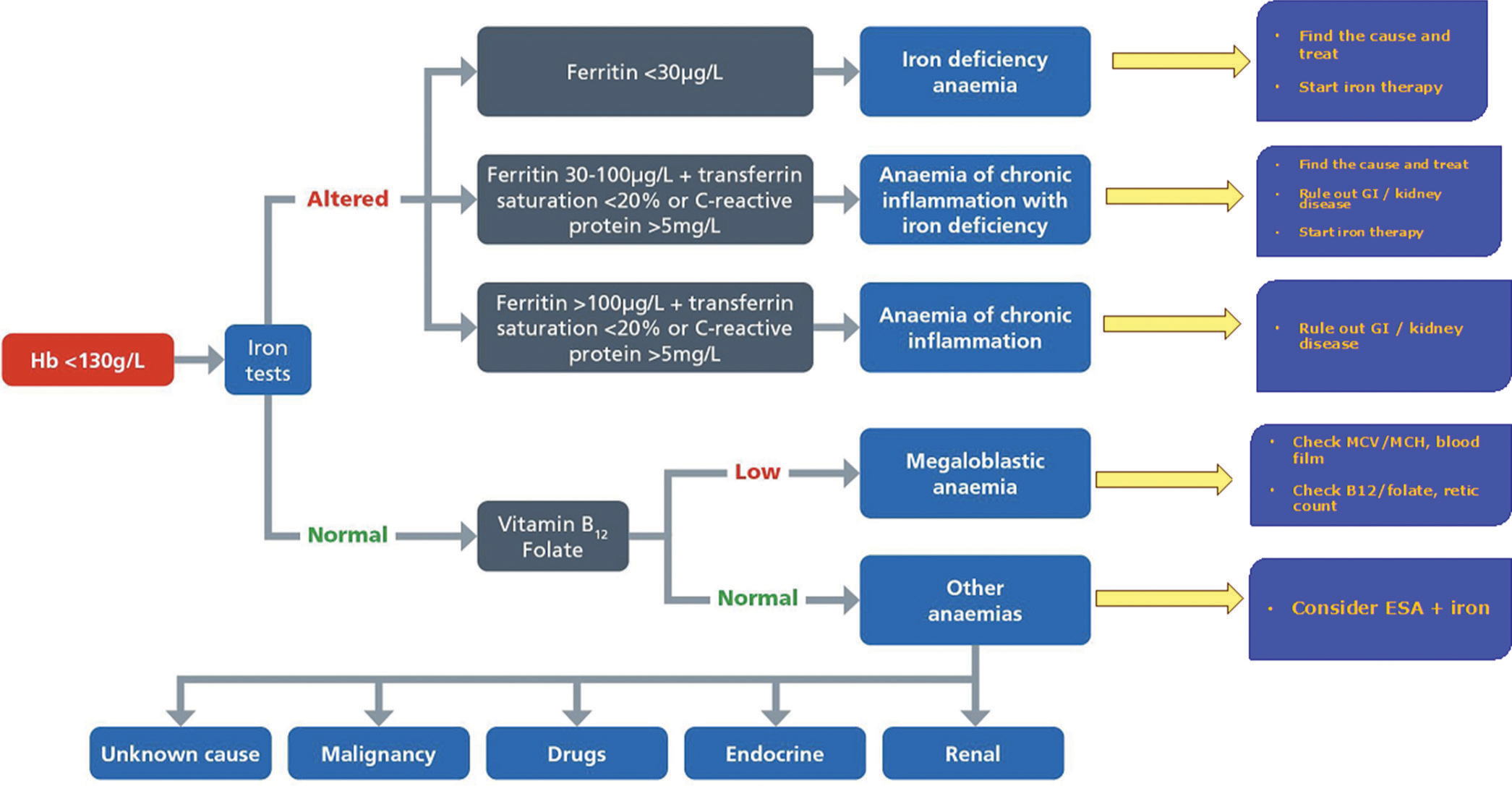
- Algorithm for Perioperative management of anemia and iron deficiency,
TRANSFUSION
Transfusion to correct preoperative anemia in anticipation of future blood loss continues. There is no evidence that transfusing patients up to near-normal hemoglobin levels is beneficial. Systematic reviews show liberal transfusion to be either inferior or non-beneficial compared to restrictive strategies. Restrictive strategies have been found to be superior to liberal transfusion in critical illness, gastrointestinal bleeding, and in elderly patients after hip fracture repair. The optimal transfusion trigger level remains to be defined and will vary for different patient groups and types of surgery. In the absence of acute myocardial or cerebrovascular ischemia, postoperative transfusion may be inappropriate for patients with a hemoglobin level of >80 g/L. There is no good evidence in support of preoperative transfusion to improve surgical outcomes or reduce total transfusion requirements.[17]
In situations where transfusion is likely to be unavoidable despite appropriate transfusion practice and intraoperative PBM (e.g., severe refractory anemia or urgent major surgery), the question of whether preoperative transfusion is superior to intraoperative transfusion is as yet unanswered.[18]
FUTURE DEVELOPMENTS
Further evaluation of “normal” Hb levels in the Asian population is necessary to avoid acceptance of low Hb levels as normal. Borderline anemia in women undergoing different surgical procedures is urgently required for optimal management of anemia in this group. At present, the CPOC guideline suggests the treatment of borderline anemia women at the discretion of local consultants.
There is a vast amount of evidence related to the benefits of optimizing red cell mass and other components of PBM. All member countries should take urgent measures to implement the principles of PBM in their day-to-day practice to improve patient safety.
CONCLUSION
All surgical patients should be evaluated as early as possible to detect preoperative anemia to minimize red blood cell transfusion, which may be associated with an increased risk of morbidity, mortality, ICU, and hospital length of stay. Optimizing red cell mass includes identification of the type and cause of anemia and initiating appropriate treatment. Routine surgery should not be done on an anemic patient. A multimodal, multidisciplinary, and patient-centered approach will lead to the successful implementation of the 1st pillar of PBM.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of Interest
The author is the coordinator of PBM committee of the College of Anesthesiologists and Intensivists of Sri Lanka and convenor of the multidisciplinary team involved in formulation of National guidelines in PBM. She has received honorarium for the Certificate course in PBM conducted by Werfen Academy India.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The Urgent Need to Implement Patient Blood Management: WHO Policy Brief Geneva: WHO; 2021.
- [Google Scholar]
- Patient Blood Management and Perioperative Anaemia. BJA Educ. 2017;17:28-34.
- [CrossRef] [Google Scholar]
- International Consensus Statement on the Perioperative Management of Anaemia and Iron Deficiency. Anaesthesia. 2017;72:233-47.
- [CrossRef] [PubMed] [Google Scholar]
- Guideline for the Management of Anaemia in the Perioperative Pathway. London: CPOC; 2022.
- [Google Scholar]
- Borderline Anaemia and Postoperative Outcome in Women Undergoing Major Abdominal Surgery: A Retrospective Cohort Study. Anaesthesia. 2020;75:210-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and Outcomes of Anemia in Cancer: A Systemic Review of the Literature. Am J Med. 2004;116(Suppl 7A):11S-26.
- [CrossRef] [PubMed] [Google Scholar]
- Anaemia in Hospitalized Patients: An Overlooked Risk in Medical Care. Transfusion. 2018;58:2522-8.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis of the Association between Preoperative Anaemia and Mortality After Surgery. Br J Surg. 2015;102:1314-24.
- [CrossRef] [PubMed] [Google Scholar]
- Patient Blood Management to Reduce Surgical Risk. BJS. 2015;102:1325-37.
- [CrossRef] [PubMed] [Google Scholar]
- Intraoperative Transfusion of 1 U to 2 U Packed Red Blood Cells is Associated with Increased 30-Day Mortality, Surgical-site infection, Pneumonia, and Sepsis in General Surgery Patients. J Am Coll Surg. 2009;208:931-7.e1-2.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative Anemia-Screening Clinics. Hematology Am Soc Hematol Educ Program. 2019;2019:570-6.
- [CrossRef] [PubMed] [Google Scholar]
- Guideline for the Laboratory Diagnosis of Functional Iron Deficiency. Br J Haematol. 2013;161:639-48.
- [CrossRef] [PubMed] [Google Scholar]
- Guideline for the Laboratory Diagnosis of Iron Deficiency in Adults (excluding pregnancy) and Children. Br J Haematol. 2022;196:523-9.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative Intravenous Iron to Treat Anaemia Before Major Abdominal Surgery (PREVENTT): A Randomised, Double-blind, Controlled Trial. Lancet. 2020;396:1353-61.
- [CrossRef] [PubMed] [Google Scholar]
- Randomised Open-Label Trial Comparing Intravenous Iron and an Erythropoiesis-stimulating Agent Versus Oral Iron to treat Preoperative Anaemia in Cardiac Surgery (INITIATE trial) Br J Anaesth. 2022;128:796-805.
- [CrossRef] [PubMed] [Google Scholar]
- British Committee for Standards in Haematology Guidelines on the Identification and Management of Preoperative Anaemia. Br J Haematol. 2015;171:322-31.
- [CrossRef] [PubMed] [Google Scholar]
- (2015)2 and NICE Quality Standards for Blood Transfusion. 2016. Available from: https://www.nice.org.uk/guidance/qs138 [Last accessed on 2024 Jan 05]
- [Google Scholar]







