Translate this page into:
Intra-aortic Balloon Pump—Current Status
Muralidhar Kanchi, MD, FIACTA, FICA, MBA, FASE, Director (academic) Narayana Institute of Cardiac Sciences, Narayana Hrudayalaya Health City, Bommasandra Industrial Area, Anekal Taluk, Bangalore 560099, Karnataka India muralidhar.kanchi.dr@narayanahealth.org kanchirulestheworld@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cardiac failure is an ever-growing public health concern confronted by both developed and developing nations of the world. Intra-aortic balloon pump (IABP) is a circulatory assist device that is used to support a failing left ventricle. IABP is designed to augment myocardial perfusion by increasing coronary blood flow during diastolic phase of the cardiac cycle and unloading the left ventricle during systolic phase. Intraoperative IABP is extremely useful when facing difficulty to wean from cardiopulmonary bypass. Until the controversy is resolved, personal experience and decision of the physician and patient circumstances may form the basis for the IABP use in a clinical setting with acute left ventricular failure.
Keywords
intra-aortic balloon pump
coronary blood flow
oxygen supply/demand
counter pulsation
Introduction
Cardiac failure is an ever-growing public health concern confronted by both developed and developing nations of the world. The contributing factors for the increasing incidence and prevalence of heart failure are demographic, socioeconomic, nutritional, and epidemiologic in nature. Increased life expectancy and continued aging of the population of this planet contribute to the epidemiologic changes in the society. The natural history of the heart failure dictates a dismal prognosis: this is despite advances in the understanding of the neurohormonal changes involved in the progression of heart failure and improvements in medical management.
The insertion of a mechanical support device must be considered when aggressive medical management fails to improve cardiac performance such that the heart can no longer provide adequate organ perfusion. The mechanical devices range from intra-aortic balloon pumps (IABPs) that can be inserted at the bedside, to left ventricular assist device (LVAD) of varying complexity, to the total artificial heart (TAH). An intensive care setting and multidisciplinary approach are essential for the insertion and use of devices to support the failing heart. This discussion focuses on the recent views on the short-term mechanical circulatory assist device called the IABP—indications and management challenges (Fig. 1).
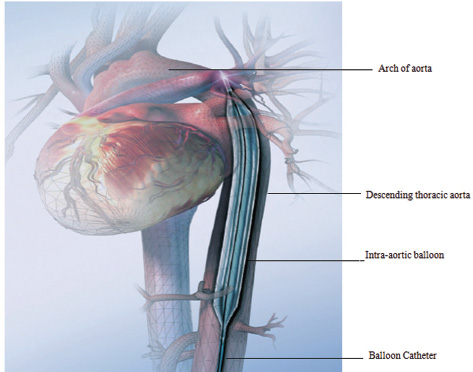
- Intra-aortic balloon in position. (Courtesy Getinge.)
The IABP is a circulatory assist device (Fig. 1). The primary effect of the IABP is to maintain cardiac output by supporting the failing left ventricle; the effect on right ventricle is indirect and not fully established. IABP is designed to augment myocardial perfusion by increasing coronary blood flow during diastolic phase of the cardiac cycle and unloading the left ventricle during systolic phase. The improvement in cardiac function with IABP is achieved by mass displacement of about 30 to 50 mL of blood by sequential inflation and deflation of a balloon positioned in the proximal segment of the descending thoracic aorta. The gas used for alternating inflation and deflation of the balloon is either helium (because of its inertial properties and rapid diffusion coefficients) or carbon dioxide (because of its great solubility in blood). The triggering of inflation and deflation of the balloon is timed synchronously with the cardiac cycle using the electronics of the balloon console to produce counter-pulsations. There is often a dramatic improvement in mean arterial pressure, ejection fraction, cardiac output, and coronary blood flow as well as decrease in arterial and ventricular systolic pressure, left ventricular end-diastolic pressure (LVEDP), pulmonary capillary wedge pressure (PCWP), left atrial pressure (LAP), heart rate (HR), and control/suppression of cardiac arrhythmias, especially atrial or ventricular arrhythmia. The hemodynamic effects of IABP are listed in the Boxes 1–3 and Fig. 2 a, b.
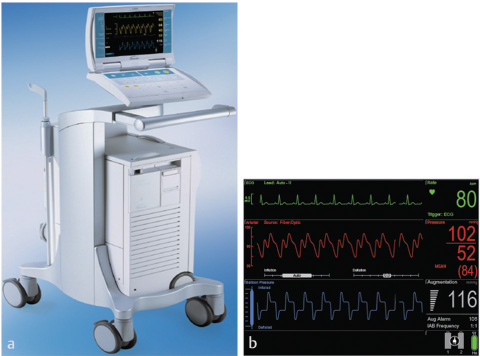
- Front view of intra-aortic balloon pump. (Courtesy Getinge.) (b) Monitor screen of the IABP. (Courtesy Getinge.)
Insertion of Intra-aortic Balloon Pump
The balloon catheter is inserted through the femoral artery either percutaneously or surgically by cut-down. During the early days of development of the IABP, insertion was done by surgical access. Alternative routes of IABP insertion included the subclavian, ulnar, brachial, and iliac arteries. IABP catheter can be inserted using transthoracic approach, especially during cardiac surgery. Subsequent improvements and refinements in IABP design allowed the use of percutaneous insertion techniques. Currently, percutaneous IABP insertion is the preferred technique and is performed rapidly bedside in intensive care units (ICUs), coronary care units, emergency departments, and operating rooms with commercially available kits. The femoral artery palpated carefully and the length of the balloon to be inserted are estimated by laying the balloon tip on the patient's chest at Louis’ angle and appropriately marking the distal point corresponding to the femoral artery. Caution is necessary while removing the balloon from its package, and it is mandatory to follow the manufacturer's instructions precisely so as not to cause perforation of the balloon before insertion. The femoral artery is accessed with the needle supplied, a J-tipped guidewire is advanced through the needle to the level of the aortic arch, and the needle is removed. The arterial puncture site is enlarged with the sequential passage of 8F dilator and then a 10.5 or 12F dilator and sheath combination. The dilator is then removed, leaving the sheath and guidewire in the artery. The balloon is threaded over the guidewire into the central aorta to the previously estimated correct position in the thoracic segment of the descending aorta. With a view to reduce risk for ischemic complication of the distal extremity, sheath is gently pulled back to connect with the leak-proof cuff on the balloon hub, so that the entire sheath is out of the arterial lumen. Other alternative is to strip the sheath off the balloon, thereby entirely removing the sheath from the insertion site. The correct position of the balloon is verified by fluoroscopy, if available, on spot or checked by radiography or echocardiography after insertion. A reasonable estimate of position of the balloon tip may be made if an indwelling left radial arterial catheter is functioning at the time of insertion, by inspecting balloon-mediated change of the arterial pulse waveform. After confirmation of the appropriate positioning and timing of the balloon, 1:1 counter-pulsation may be initiated. The procedure of insertion is performed under strict aseptic precautions, and the entire external balloon assembly is covered in sterile dressings (Fig. 3).
- Intra-aortic balloon (IAB) as seen from rear. (Courtesy Getinge.)
Removal of a percutaneously inserted IABP may be done by the open (surgical removal) or closed technique. Irrespective of the technique chosen, steps are taken to prevent distal embolization of clot. If surgical removal is chosen, embolectomy catheters may be passed antegrade and retrograde before suture closure of the artery. If a closed technique is opted for, the artery should be allowed to bleed for several seconds while maintaining pressure on the distal artery after balloon removal; this is to flush any accumulated clot from the central lumen. Pressure is then applied for 20 to 30 minutes on the puncture site to achieve hemostasis. IABP can be inserted using alternative routes. In patients with extreme peripheral vascular disease or in pediatric patients in whom the peripheral vasculature is too small, IABP can be inserted through the ascending aorta or aortic arch. This approach is suitable if the chest is open; if not, this will necessitate median sternotomy for insertion and usually require reexploration for removal. Other routes of access for insertion of IABP include the abdominal aorta and the subclavian, axillary, and iliac arteries. The iliac artery approach may be especially useful for pediatric patients. Procedure to be followed for sheath less insertion of IABP is shown in a stepwise manner in the following text:
Preparing the Intra-aortic Balloon Catheter and Sheathless Insertion
Steps in preparing intra-aortic balloon (IAB) catheter and its sheathless insertion are shown in Fig. 4.
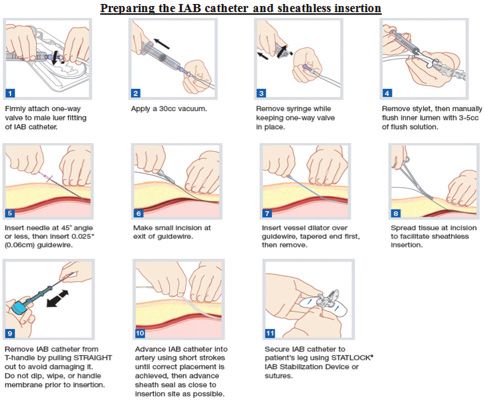
- Steps in preparation and sheathless insertion of intra-aortic balloon (IAB) catheter. (Courtesy Getinge.)
Timing for Balloon Inflation
A variety of IABP systems from diverse manufacturers are commercially available. The basic design of the console includes monitoring of electrocardiography, arterial blood pressure waveform, balloon volume monitoring, switches for triggering selection, adjustments for inflation and deflation timing, battery backup, power source, gas reservoir, and option for printing. Recently, IABP systems have become quite refined with advanced computer microprocessor circuits; these circuits allow triggering based on pacemaker signals or detection/compensation for aberrant rhythms such as atrial fibrillation. Portable models of IABP are now available, which can be used for transportation of patients by ground, helicopter, or air ambulances. It is mandatory that inflation and deflation of the balloon be adjusted accurately and timed precisely to the cardiac cycle for optimal effect of the IABP. The performance of the IABP is affected by a number of variables, such as positioning of the balloon within the aorta, balloon volume, and the patient's cardiac rhythm. Balloon inflation should be in synchrony with closure of aortic valve (AV); if this is not so, aortic insufficiency and left ventricular (LV) strain will result. In the same way, delayed inflation will cause a diminished coronary perfusion pressure. Premature deflation will cause inappropriate loss of afterload reduction, and late deflation will increase LV work by causing increased afterload, if only transiently. The correct timing and errors are shown diagrammatically in Figs. 5 6.
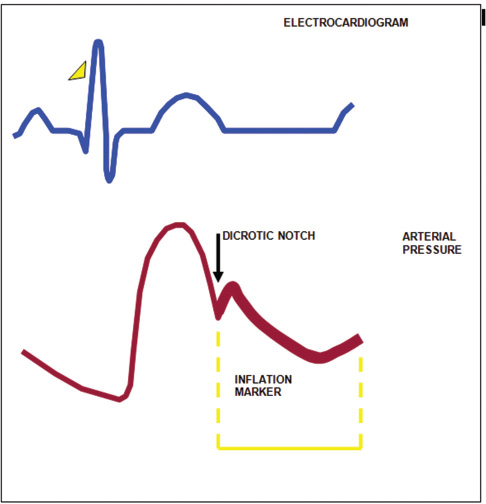
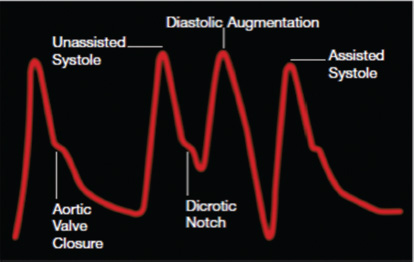
- The balloon is inflated during diastole, and inflation is timed to coincide with dicrotic notch of arterial waveform.
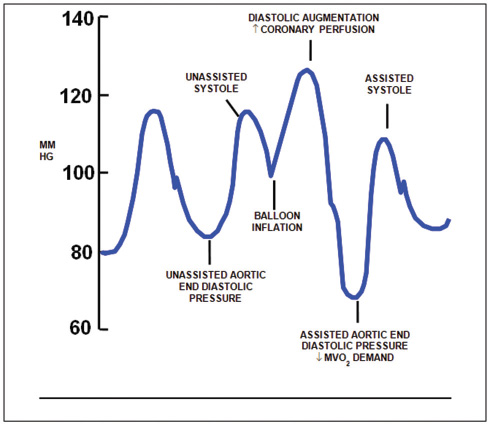
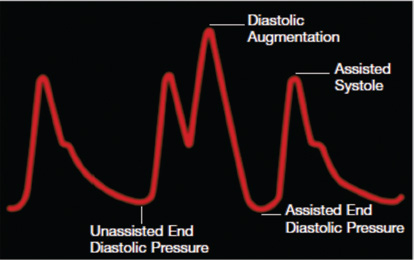
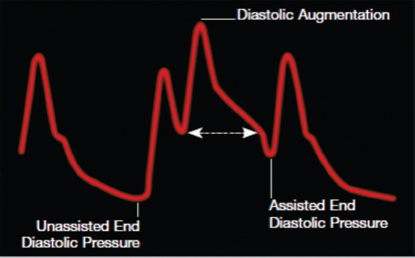
- Arterial waveform variations during IABP therapy. Arterial pressure is shown on the vertical axis.
-
Inflation: Inflation is simply the expansion of the balloon catheter with helium, which is timed to just after the closure of the AV. This is shown on the arterial waveform as the dicrotic notch. The dicrotic notch denotes closure of the AV, when blood has been ejected from the (LV) ventricle into the aorta. If inflated correctly, a V shape should be shown on the balloon trace. The effect of displacement of blood in the aorta causes an increase in diastolic arterial pressure and an increase in cardiac output.
-
Deflation: Deflation is the collapse of the balloon and the transfer of helium back into the console. Deflation of the IAB occurs in systole. Deflation is typically seen on the screen as being half way down the slope after the dicrotic notch, prior to the AV opening. The effect is a decrease in aortic end-diastolic pressure (afterload) by the balloon deflating and creating space in the aorta. This causes less impedance to blood being ejected from the left ventricle during systole when the balloon is deflated resulting in a reduced ventricle wall tension, increased stroke volume, and improvement in myocardial performance (Fig. 7).
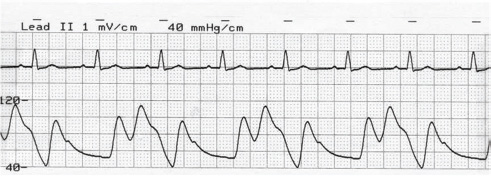
- Optimal timing of inflation of IABP balloon. The arterial pressure tracing and corresponding ECG is shown. Note inflation during diastole at the rate of 1:2.
Timing Errors in Intra-aortic Balloon Pump
-
Early inflation: Inflation of the IAB prior to AV closure (Fig. 8)
2. Late inflation: Inflation of the IAB markedly after closure of the AV (Fig. 9).
3. Early deflation: Premature deflation of the IAB during the diastolic phase will lead to the following (Fig. 10).
4. Late deflation: Deflation of the IAB late in diastolic phase as AV is beginning to open (Fig. 11).
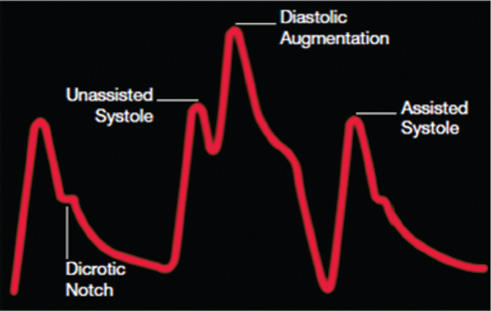
- Arterial tracing with early inflation. Early inflation occurs when the balloon inflates prior to aortic valve closure; then there will be insufficient blood in the aorta, resulting in a decrease coronary blood flow, premature closure of aortic valve, and increased afterload.
- Arterial tracing with late inflation. Late inflation occurs when the balloon inflates too late after the closure of the aortic valve and blood escapes down the aorta to the rest of the body, instead of being directed to the coronary arteries.
- Arterial tracing with early deflation. Early deflation causes retrograde blood flow to the coronary arteries and affecting forward blood flow to other vessels and increase in afterload.
- Arterial tracing with late deflation. Late deflation has the effect of impeding left ventricular ejection, causes ventricular wall stress, and impedes afterload reduction.
Weaning Intra-aortic Balloon Pump
When the patient is hemodynamically stable and it is evident that LV support is no longer required, weaning of the IABP is commenced. Judicious intravenous infusion and dosing of vasodilator and inotropic medications can assist this procedure. The balloon augmentation may be reduced in steps from 1:1 counter-pulsation to 1:2 and then to 1:4, with appropriate intervals at each stage to assess changes in hemodynamic and neurologic stability, cardiac output, and mixed venous oxygen saturation. After appropriate observation at 1:4 or 1:8 counter pulsation, balloon assistance can be safely discontinued, and the device can be removed by one of the methods described above. If percutaneous removal is chosen, an appropriate interval for reversal of anticoagulation (if used) before removal of the balloon should be allowed.
Balloon Sizing in Intra-aortic Balloon Pump
The balloon size is dependent on the patient's height. This is to prevent occlusion of left subclavian or renal arteries. Selection of balloon based on patient height is shown in Fig. 12.
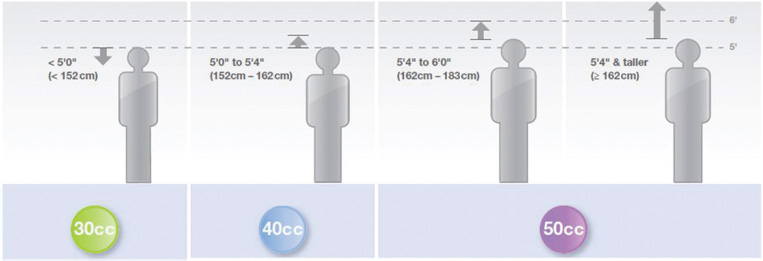
- Selection of balloon based on patient height. (Courtesy Maquet-Getinge group: Intra-aortic balloon reference sizing guide.)
IABP Triggers
|
1. Electrocardiogram (ECG) |
Uses R wave on the ECG to time the inflation. |
|
2. Pressure |
The arterial pressure waveform is used to trigger. |
|
3. Internal |
This allows a trigger set at 80 beats/min. Internal mode should never be used if a patient is generating a cardiac output useful to generate pulsatile flow during cardiopulmonary bypass (CPB) |
|
4. Ventricular/atrioventricular pacing |
Uses ventricular spike to trigger an event, is not an appropriate trigger for demand pacing. |
|
5. Atrial pacing |
The R wave on the ECG is the trigger; the atrial pacer spikes are enhanced and rejected. This mode is only used if ECG trigger is not able to interpret R wave with A pacing. Never use if patient is ventricularly paced. |
Steps to Set up the Intra-aortic Balloon Pump to Commence Counter-Pulsation
-
Connect to mains power to ensure the battery is preserved. The battery can run the IABP for approximately 2 hours (depending on the battery charge).
-
Check the helium tank is open at the back of the pump.
-
Ensure both an ECG and pressure trace can be obtained from the patient on the screen of the IABP. This can be obtained from either direct monitoring or the use of the slave cables taking the signal from the monitor.
-
Obtain a trigger for IABP and set optimal timings, which initiates balloon inflation.
-
Frequency of inflation when first commencing IABP is 1:1, which means that for each heartbeat, the balloon will be inflated.
-
To commence balloon counter pulsation, inflation and deflation points should be set, and then after IABP is established, timing should be reassessed.
-
Connect the extension tubing to the balloon catheter and on the balloon console at the back. Once connected, press the IAB fill button, holding it down for a second. A prompt on the screen will come up, so you know it is filling.
-
Once filled, commence counter-pulsation by pressing the assist/standby button. Then increase slowly the augmentation to maximum. This will unwrap the balloon; if done too fast, the balloon may unwrap incompletely. Maximum augmentation is maintained at all times to ensure that the balloon is fully inflated and blood clots are not able to form beside the balloon or in wrapping (Table 1).
|
Vascular |
Balloon related |
Miscellaneous |
|---|---|---|
|
Abbreviation: IABP, intra-aortic balloon pump. |
||
|
Lower extremity ischemia |
Perforation (pre-insertion) |
Infection |
|
Arterial injury (perforation, dissection) |
Tear (during insertion) |
Hemorrhage |
|
Aortic perforation |
Incorrect positioning |
Hemolysis |
|
Aortic dissection |
Left internal mammary artery occlusion |
Thrombocytopenia |
|
Femoral artery thrombus |
Gas embolization |
Claudication (post-removal) |
|
Peripheral embolization |
Inadvertent removal |
|
|
Femoral vein cannulation |
Entrapment |
|
|
Pseudoaneurysm of femoral vessels |
||
|
Spinal cord necrosis |
||
|
Paraplegia |
||
|
Compartment syndrome |
||
|
Visceral ischemia |
||
Intra-aortic Balloon Pump for Myocardial Infarction with Cardiogenic Shock
Use of IABP for treatment of cardiogenic shock was considered as class I indication. However, evidence for this recommendation was mainly based on data from registry.12 In a randomized, prospective, open-labeled multicenter trial (Should we emergently revascularise Occluded Coronaries for cardiogenic shock [SHOCK] II trial), a total of 600 patients with cardiogenic shock complicating acute myocardial infarction (MI) were assigned to IABP or no IABP; 300 patients in the IABP group and 298 in the control group were included in the analysis of the primary endpoint that was defined as 30-day all-cause mortality.13 At 30 days, 119 (39.7%) patients in the IABP group and 123 (41.3%) patients in the control group had died. There were no significant differences in secondary endpoints or in process-of-care measures, including the time to hemodynamic stabilization, the length of stay in the ICU, serum lactate levels, the dose and duration of catecholamine therapy, and renal function. The IABP and the control groups did not differ significantly about the rates of major bleeding, peripheral ischemic complications, sepsis, and stroke. Although the study suffered from the limitations of small size and cross-over design, this study results challenged the routine use of IABP for cardiogenic shock. Based on this trial results, the European Society of Cardiology (ESC) has downgraded the use of IABP in ST-segment elevation MI from 1C to 2B.1. ESC guidelines say that IABP may be considered for hemodynamic support in selected patients (i.e., severe mitral insufficiency or ventricular septal defect). There has been contradicting evidences regarding the benefits of use of IABP in MI patients. Zheng et al,14 on assessment of seven trials conducted from 1999 to 2013, concluded that IABP reduced 30-day and 6-month mortality when used in MI patients without cardiogenic shock. Use of IABP in MI with cardiogenic shock was noted as not beneficial. Wan et al15 noted similar findings with no beneficial effects with use of IABP in terms of long-term and short-term mortality in MI patients irrespective of the presence of cardiogenic shock. They analyzed 12 randomized controlled trials (RCTs) from 1994 to 2012, including SHOCK I and SHOCK II trials. Even though the beneficial effects of use of IABP are debatable, there was no adverse effect on long-term mortality following the use of the same. SHOCK II trial follow-up in 2018 showed that despite that there was no benefit on the primary outcome, there were no important safety concerns from the use of IABP.
Intra-aortic Balloon Pump in Cardiac Surgery
Intra-aortic balloon pump is currently used extensively in cardiac surgery for the support of failing left ventricle. In a study of a cohort of 2,697 adult cardiac surgical patients undergoing open-heart surgery, 136 (5.04%) patients required IABP.16 The overall operative mortality in those receiving IABP was 35.3%. The incremental risk factors for death included female sex, smoking, preoperative creatinine > 120 μmol/L, aortic cross clamp time > 80 minutes, and IABP insertion postoperatively. The incremental risk factors for the development of complications included poor ejection fraction, Euroscore > 7, and history of peripheral vascular disease. In a recent study, it was shown that IABP is safe with no major complications, but composite endpoint of acute MI (AMI), acute kidney injury (AKI), cerebrovascular accident (CVA), and in-hospital mortality occurred in 44% of patients who received IABP.17 Obviously, the benefit of coronary artery bypass grafting (CABG) in terms of improvement in quality of life was lost in patients who suffered the composite endpoint. The effect of prophylactic IABP was variable, and this calls for an RCT on the use of prophylactic IABP in high-risk CABG.
The benefits of IABP in patients undergoing cardiac surgery are well established. Ding et al18 assessed the benefits of IABP in 116 patients who underwent cardiac surgery, and concluded that there is significant improvement in cardiac performance and reduction in respiratory and renal complications, all of which contributes to reduction in mortality. This finding was supported by Wang et al,19 who performed a meta-analysis of 17 studies, including 2,539 patients who underwent CABG, and this is the first meta-analysis to demonstrate an improvement in renal function without any significant IABP-related complications.
Intra-aortic Balloon Pump in Cardiac Surgery at Naryana Hrudayalaya
In a cardiac surgical cohort of 4,500 adult patients, the incidence of use of IABP was 4.5%, and the most common indication was myocardial ischemia with hemodynamic instability (80%). Mean duration of IABP use was 79 hours (range: 5–432 hours), site of insertion was invariably femoral artery, and mortality rate was 28% (Table 2).
|
Abbreviations: CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; IABP, intra-aortic balloon pump; VSR, ventricular septal rupture. |
|
|
Male/Female |
85/15 |
|
Incidence of insertion in adult cardiac surgery |
100/4500 = 2.02% |
|
Indication |
Myocardial Ischemia with cardiac instability (n = 72) Difficulty in weaning -CPB (n = 8) Cardiac arrest (n = 8) Intractable arrhythmia (n = 5) VSR (n = 2) Pulmonary edema (n = 1) Cardiogenic shock (n = 4) |
|
Surgery |
Isolated CABG (n = 80) CABG + combined (n = 12)_Non-CABG (n = 8) |
|
Site of insertion |
Femoral artery = 100% |
|
Timing of IABP (Preoperative/Intraoperative/Postoperative) |
3/62/35 |
|
Technique |
Percutaneous = 99% |
|
Complications |
11 (Vascular 7% + Balloon-related 4%) |
|
Duration |
5–341 h (mean = 80.2h) |
|
Mortality |
28% |
Conclusion
Intra-aortic balloon pump is the most commonly used LV assist device used in medical practice today. IABP counter-pulsation increases coronary blood flow during diastole and unloads the left ventricle by decreasing the afterload during systole. These effects are helpful to support a failing left ventricle. The ESC no longer strongly recommends the use of IABP in patients with MI associated cardiogenic shock. In a cardiac surgical setting, preoperative insertion of IABP is often helpful in unstable angina, left main disease with ongoing ischemia, and ischemia leading to ventricular arrhythmia. For patients with mechanical complications of AMI, such as acute mitral regurgitation or ventricular septal rupture, insertion of IABP may be lifesaving. Likewise, intraoperative IABP is extremely useful when facing difficulty to wean from CPB. Until the controversy regarding the long-term benefits of the use of IABP is resolved, personal experience and decision of the physician and patient circumstances may form the basis for the IABP use in a clinical setting with acute left ventricular failure.
Conflict of Interest
None.
References
- Basic principles of the intraaortic balloon pump and mechanisms affecting its performance. ASAIO J. 2005;51(3):296-300.
- [Google Scholar]
- Aortic counterpulsation: a review of the hemodynamic effects and indications for use. Catheter Cardiovasc Interv. 2006;67(1):68-77.
- [Google Scholar]
- Preoperative intra aortic balloon pumps in patients undergoing coronary artery bypass grafting. Cochrane Database Syst Rev (1):CD004472.
- [Google Scholar]
- Preoperative intra-aortic balloon pump in patients undergoing coronary bypass surgery: a systematic review and meta-analysis. J Card Surg. 2008;23(1):79-86.
- [Google Scholar]
- Preoperative intraaortic balloon pumping improves outcomes for high-risk patients in routine coronary artery bypass graft surgery. Ann Thorac Surg. 2009;87(2):481-488.
- [Google Scholar]
- Reduced mortality in high-risk coronary patients operated off pump with preoperative intraaortic balloon counterpulsation. Ann Thorac Surg. 2007;84(2):498-502.
- [Google Scholar]
- Effects of intra-aortic balloon pumping on graft flow in coronary surgery: an intraoperative transit-time flowmetric study. Ann Thorac Surg. 2008;86(3):823-827.
- [Google Scholar]
- Intra-aortic balloon pumping: effects on left ventricular diastolic function. Eur J Cardiothorac Surg. 2003;24(2):277-282.
- [Google Scholar]
- Beat-to-beat effects of intraaortic balloon pump timing on left ventricular performance in patients with low ejection fraction. Ann Thorac Surg. 2005;79(3):872-880.
- [Google Scholar]
- Paraplegia complicating intraaortic balloon pumping. Ann Thorac Surg. 1997;63(4):1217-1218.
- [Google Scholar]
- Vascular complications of the intraaortic balloon pump in patients undergoing open heart operations: 15-year experience. Ann Thorac Surg. 1999;67(3):645-651.
- [Google Scholar]
- Intra-aortic balloon pump for high-risk percutaneous coronary intervention. Circ Cardiovasc Interv. 2014;7(5):712-720.
- [Google Scholar]
- Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287-1296.
- [Google Scholar]
- The effectiveness of intra-aortic balloon pump for myocardial infarction in patients with or without cardiogenic shock: a meta-analysis and systematic review. BMC Cardiovasc Disord. 2016;16(1):148-159.
- [Google Scholar]
- The effects of intra-aortic balloon pumps on mortality in patients undergoing high-risk coronary revascularization: a meta-analysis of randomized controlled trials of coronary artery bypass grafting and stenting era. PLoS One. 2016;11(1):e0147291.
- [Google Scholar]
- The need for intra aortic balloon pump support following open heart surgery: risk analysis and outcome. J Cardiothorac Surg. 2010;5:20.
- [Google Scholar]
- Six-month outcomes after high-risk coronary artery bypass graft surgery and preoperative intra-aortic balloon counterpulsation use: an inception cohort study. J Cardiothorac Vasc Anesth. 2018;32(5):2067-2073.
- [Google Scholar]
- Prophylactic application of an intra-aortic balloon pump in high-risk patients undergoing off-pump coronary artery bypass grafting. Cardiology. 2015;131(2):109-115.
- [Google Scholar]
- Preoperative prophylactic intraaortic balloon pump reduces the incidence of postoperative acute kidney injury and short-term death of high-risk patients undergoing coronary artery bypass grafting: a meta-analysis of 17 studies. Ann Thorac Surg. 2016;101(5):2007-2019.
- [Google Scholar]






