Translate this page into:
Extracorporeal Membrane Oxygenation for Cardiotoxic Drug Overdose—A Lifesaving Intervention
Sundar Singh Subash, MD, DM, FTEE, FIAE Department of Cardiac Anaesthesia, Kerala Institute of Medical Sciences PB No: 1, Anayara, Trivandrum, Kerala, 695029 India sssubash@yahoo.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Extracorporeal membrane oxygenation (ECMO) use in cardiotoxic drug overdose is increasing. Calcium channel blockers along with β-blockers constitute more than 65% of deaths from cardiovascular medications. The authors report here a case of ECMO use for amlodipine and telmisartan tablet overdose, where ECMO was used to salvage the patient’s life as a life-saving intervention.
Keywords
calcium channel blocker poisoning
angiotensin receptor antagonist poisoning
extracorporeal membrane oxygenation
refractive shock
Introduction
Calcium channel blocker (CCB) constitutes 34% of cardiovascular drug-related deaths.1 When multiple cardiovascular drugs with synergistic mechanisms are ingested together, the management of such patients becomes more challenging and refractive to most medical management. Extracorporeal membrane oxygenation (ECMO) can become a lifesaving intervention in such a situation, when not responding to other medical management.
Case Report
A 28-year-old female patient was brought to the hospital with alleged history of consumption of amlodipine 300 mg and telmisartan 120 mg, in a suicide attempt. She was brought to the hospital after 5 hours of ingestion of these tablets. At the time of admission, she was conscious, oriented, and responding to verbal commands. Her vitals were as follows: heart rate 110 beats/min, blood pressure (BP) 80/50 mm Hg, and Spo2 98% at room air. Her cardiovascular and respiratory examinations were normal.
The patient was initially managed with activated charcoal via nasogastric tube, normal saline infusion bolus, Inj. noradrenaline started at 0.1 μg/kg/min, and Inj. calcium gluconate. She was shifted to intensive care unit, and her BP continued to decrease. Inj. noradrenaline increased to 0.5 μg/kg/min, and Inj. adrenaline 0.5 μg/kg/min, Inj. vasopressin 4.8 U/h, and Inj. phenylephrine 14 μg/kg/min were also added. Inj. glucagon infusion at 4 mg/h, 20% smof lipid 100 mL, and calcium gluconate 10% infusion at 10 mL/h were also initiated. Considering the amlodipine poisoning, hyperinsulinemic-euglycemic mixture was also tried. In spite of these aggressive medical managements, her BP never showed any improvement. The patient was intubated considering her hemodynamic disturbances. Her arterial blood gas (ABG) showed severe metabolic acidosis with PH 7.10, and she went into severe vasoplegic state with acute renal failure. Transthoracic echocardiography showed good left ventricular function, normal functioning valves, and ejection fraction 60%. Considering her failure to show any improvement for aggressive medical management, the decision was made to support the patient with VA ECMO.
The VA ECMO circuit consisted of Rotaflow centrifugal pump, Quadrox oxygenator, 22F venous cannula in the right femoral vein, 17F arterial cannula in the left femoral artery, and 10F arterial cannula in the same artery for distal perfusion. The cannulas were placed under transesophageal echocardiographic guidance (Fig. 1 Fig. 2 Fig. 3 Fig. 4). The flow was maintained at 4.2 L/min and sweep gas flow rate of 3L/min with Fio2100%. Activated clotting time was maintained between 180 and 200 seconds with heparin infusion, and the patient was ventilated with synchronous intermittent mandatory ventilation (SIMV) mode with tidal volume 200 mL, respiratory rate10 breaths/min, positive end-expiratory pressure 7 mm Hg, and Fio2 35%. She was supported with VA ECMO for 5 days, and vasopressors and inotropes were gradually weaned with ongoing VA ECMO support. Her laboratory values were shown in Table 1. On fifth day, she was successfully weaned from VA ECMO and decannulated. The following day, she was weaned from ventilator and extubated. She was discharged on 12th day, and follow-up after 2 months showed favorable outcome.
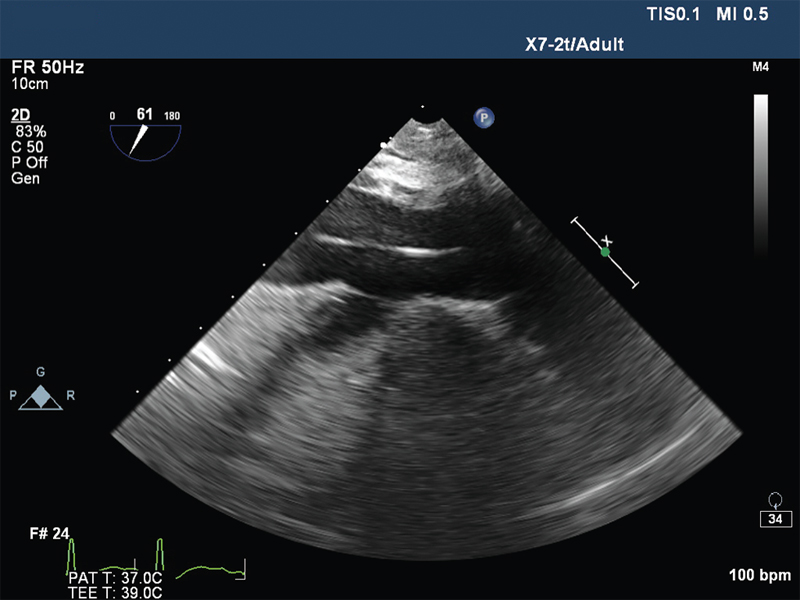
- Hepatic vein view showing guide wire in inferior vena cava.
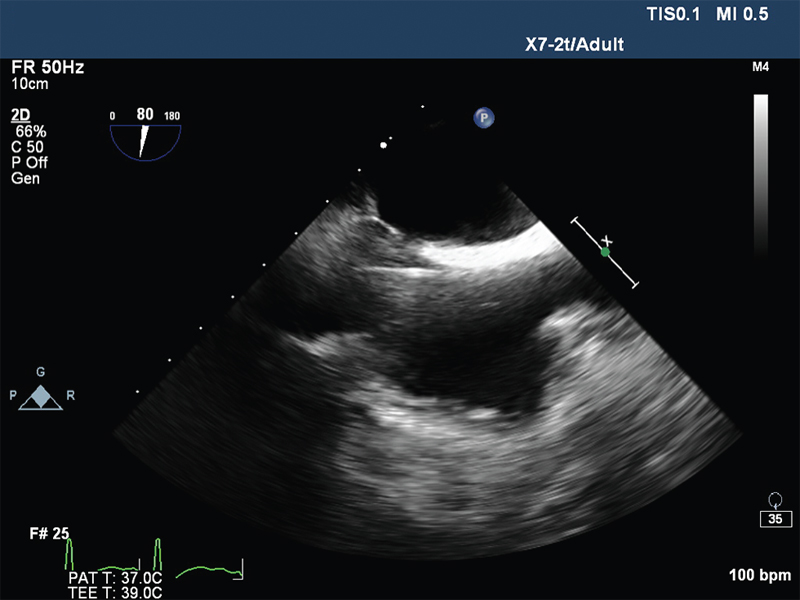
- Mid oesophageal bicaval view showing guide wire in right atrium.
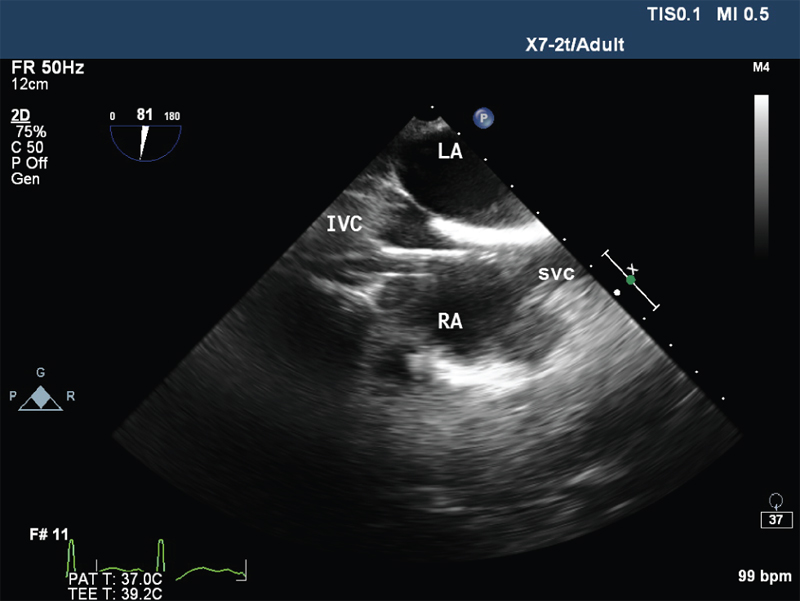
- Mid oesophageal Bicaval view showing venous cannula in right atrium. IVC , inferior vena cava; LA, left atrium; RA, right atrium; SVC, superior vena cava.
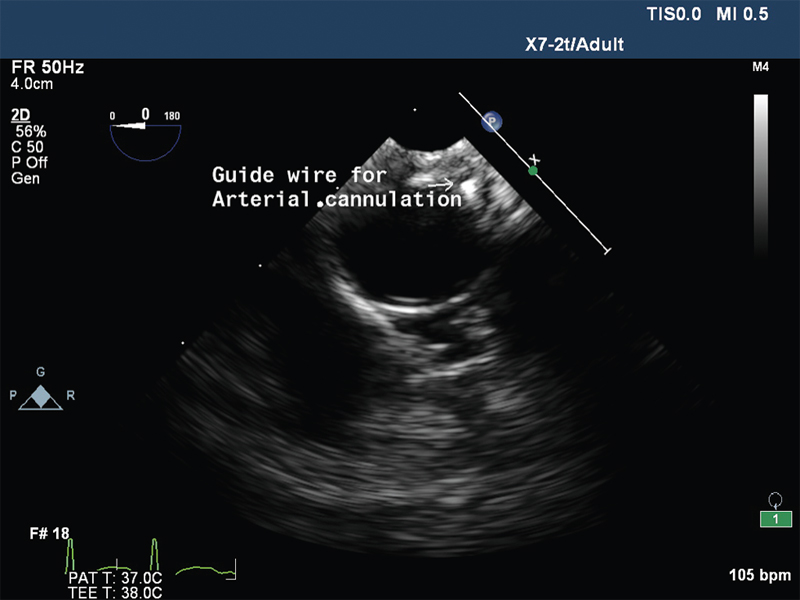
- Descending thoracic aorta short axis view showing guide wire.
|
Days |
Hb (g/dL) |
TLc (cells/mm3) |
Platelets (thous/mm3) |
Na+ (mEq/L) |
K+ (mEq/L) |
Urea (mg/dL) |
Creatinine (mg/dL) |
|---|---|---|---|---|---|---|---|
|
1 |
13.2 |
39,600 |
342 |
149 |
3.8 |
7.7 |
1.6 |
|
2 |
14.5 |
40,000 |
207 |
149 |
2.5 |
9.0 |
0.8 |
|
3 |
13.2 |
48,100 |
128 |
143 |
5.1 |
7.0 |
0.8 |
|
4 |
12.0 |
42,500 |
88 |
138 |
5.7 |
14.4 |
1.4 |
|
5 |
10.9 |
19,000 |
64 |
143 |
4.0 |
18.6 |
1.4 |
|
6 |
12 |
10,900 |
79 |
145 |
4.0 |
17.9 |
1.4 |
Discussion
CCB and angiotensin receptor blocker overdose can cause serious mortality and morbidity. Half-life of amlodipine and telmisartan are 34 to 50 hours and 24 hours, respectively.2 The overdose of these drugs can cause profound refractory hypotension, bradyarrhythmias, and shock. The combination of these drugs may blunt the sympathetic and vasoconstrictive responses and worsen CCB toxicity.3 These drugs are not dialyzable because they are highly protein bound with large volume of distribution.4
The medical management of CCB drug overdose includes general and specific measures. General measures include stomach wash via nasogastric tube with activated charcoal. Special measures include administration of vasoconstrictors, intravenous calcium gluconate, and Inj. glucagon that acts by increasing intracellular cyclic adenosine monophosphate (cAMP). Hyperinsulinemia-euglycemia therapy involves infusion of high dose of insulin along with 25% dextrose to maintain euglycemia. Insulin has positive inotropic action, increases plasma levels of ionized calcium, and improves the myocardial use of carbohydrates instead of free fatty acids.5 Lipid emulsion forms an expanded lipid phase in blood and helps in redistribution and trapping of tissue bound drug.6 Methylene blue helps in scavenging the nitric oxide.7 In this patient, all these drugs were used except methylene blue because of nonavailability of the drug, and these medical managements did not show any improvement.
Toxicology consultation has shown that ECMO is still rarely used in poisoned patients; 10 out of 26,271 patients were reported over a 3-year period.8 Because this patient did not show any improvement to medical management along with refractory shock and acute renal failure, the authors decided to go ahead with VA ECMO. The goal of the VA ECMO was to temporarily support the hemodynamics and respiration until the drug got metabolized from her body. Even though ECMO has disadvantages such as not readily available and high cost, it can be a lifesaving intervention when medical management fails.
Conclusion
When medical therapy is ineffective for cardiotoxic drug overdose, ECMO may be a lifesaving intervention.
References
- 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th Annual Report. Clin Toxicol (Phila). 2012;50(10):911-1164.
- [Google Scholar]
- Prolonged severe hypotension following combined amlodipine and valsartan ingestion. Clin Toxicol (Phila). 2008;46(5):470-474.
- [Google Scholar]
- Pharmacology, pathophysiology and management of calcium channel blocker and beta-blocker toxicity. Toxic Rev. 2004;23:223-238.
- [Google Scholar]
- Insulin-glucose as adjunctive therapy for severe calcium channel antagonist poisoning. J Toxicol Clin Toxicol. 1999;37(4):463-474.
- [Google Scholar]
- Intravenous fat emulsion: a potential novel antidote. J Med Toxicol. 2008;4(2):109-114.
- [Google Scholar]
- Vasoplegic syndrome—the role of methylene blue. Eur J Cardiothorac Surg. 2005;28(5):705-710.
- [Google Scholar]
- Survival after cardiac arrest: ECMO rescue therapy after amlodipine and metoprolol overdose. Cardiovasc Toxicol. 2017;17(2):223-225.
- [Google Scholar]






