Translate this page into:
Cardiac Surgery in a Patient with Implanted Brain Pacemaker: A Case Report
Ajmer Singh, MD Department of Cardiac Anesthesia, Institute of Critical Care and Anesthesiology, Medanta-The Medicity Sector-38, Gurgaon 122001, Haryana, India ajmersingh@yahoo.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Parkinson’s disease, a neurodegenerative disorder, affects approximately 1% of population older than 60 years. Patients with Parkinson’s disease with intractable symptoms often require placement of deep brain stimulator, conventionally known as brain pacemaker. Patients implanted with this device pose specific challenges to the anesthesiologist in view of the primary illness, possible drug interactions, risk of cerebral hemorrhage from anticoagulant therapy, and potential for device malfunction by electromagnetic interference during cardiac surgery. The authors describe perioperative management of a patient with implanted brain pacemaker who underwent aortic valve replacement at their institution.
Keywords
aortic stenosis
critical stenosis
deep brain stimulator
brain pacemaker
Parkinson’s disease
aortic valve replacement
pacemaker
Introduction
Patients with Parkinson’s disease (PD) who have intractable symptoms often require placement of deep brain stimulator, conventionally known as brain pacemaker. Patients implanted with this device pose specific challenges to the anesthesiologist in view of the primary illness, multisystemic involvement, possible drug interactions, risk of cerebral hemorrhage from anticoagulant therapy, and potential for device malfunction by electromagnetic interference during cardiac surgery. We describe anesthetic considerations and perioperative management of an interesting case with implanted brain pacemaker who underwent aortic valve replacement at our institution.
Case Report
A 57-year-old man with bicuspid aortic valve and severe aortic stenosis (aortic valve area 0.7 cm2, peak/mean pressure gradients across the aortic valve 89/55 mm Hg) was admitted for aortic valve replacement. He was a known case of PD who underwent bilateral subthalamic nucleus (STN) deep brain stimulator (DBS) implantation (Medtronic Activa RC, Medtronic Hong Kong Medical Ltd.) in 2016 for intractable symptoms resulting from PD. The symptoms at the time of DBS implantation included excessive tremors, dragging of right lower limb while walking, slurring of speech, and sleep disturbance resulting from PD. At the time of current admission, the patient was conscious, well-oriented, with normal power (grade 5/5) in all four limbs, decreased volume of speech with no slurring, no rigidity, and occasional tremors in the right upper limb. Current medications included levodopa, clonazepam, and escitalopram. Neurologic assessment was unremarkable except for the presence of mild tremors. Integrity of the entire system consisting of neurotransmitter/implantable pulse generator (IPG), leads, electrodes, and battery status was checked by placing the programmer over the IPG by the neurologist. Pulmonary function tests were suggestive of mild restrictive pattern. Incentive spirometry and breathing exercises were explained and commenced preoperatively. Chest radiograph showed IPG device in the left infraclavicular region with the leads going toward left side of the neck (Fig. 1).
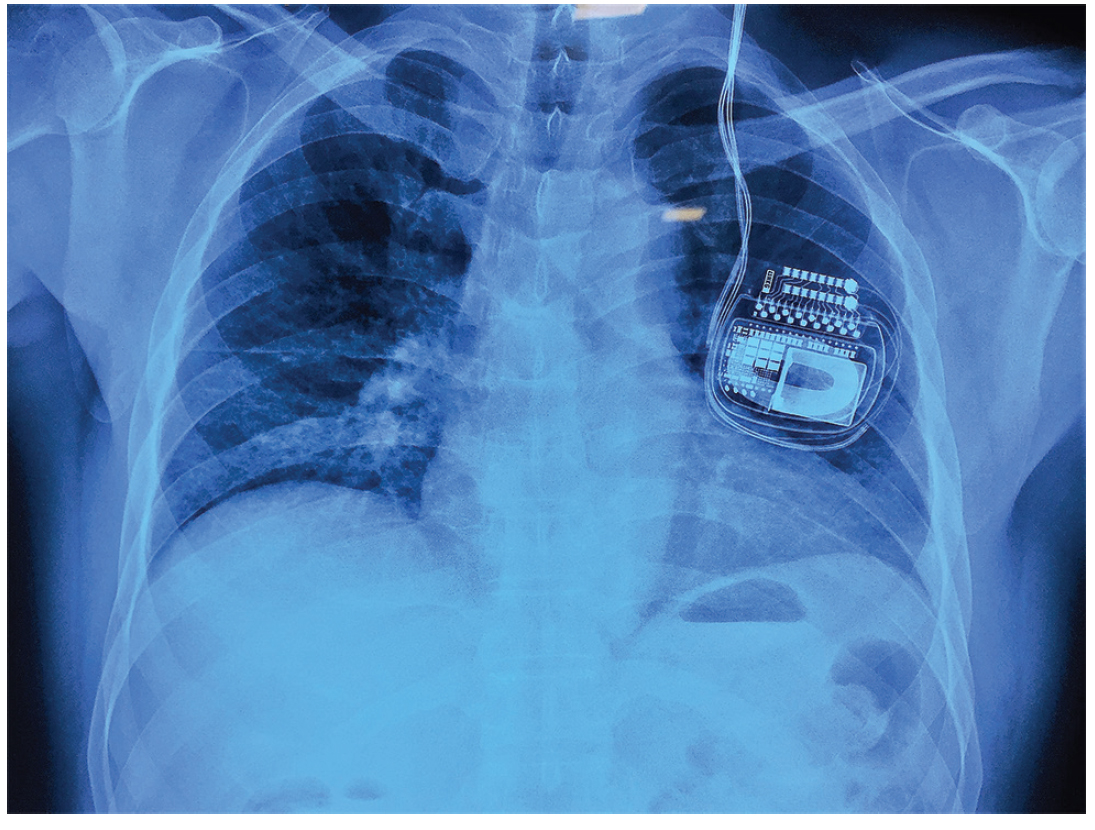
- Chest radiograph showing implanted pulse generator with extension leads.
Just before induction of anesthesia, the IPG device was turned off by using the programmer, and standard cardiac monitoring and bispectral index monitoring were commenced. A doughnut magnet was placed over the IPG device under sterile conditions. Electrocautery pad was placed under the right buttock, and bipolar cautery was used to achieve hemostasis during surgery. Aortic valve was replaced with bioprosthetic valve under hypothermic cardiopulmonary bypass using antegrade and retrograde cardioplegia. Return of cardiac activity was spontaneous with smooth weaning from cardiopulmonary bypass. Epsilon aminocaproic acid was used to reduce perioperative bleeding as an institutional protocol. After completion of the surgery, the IPG device was switched on and re-interrogated to restore original settings in the intensive care unit. Further course of the patient in the hospital, including his neurologic status, was unremarkable. Postoperative anticoagulation therapy consisted of aspirin and Coumadin aimed to keep international normalized ratio of 1.5 to 2.5 for 3 months, followed by aspirin alone.
Discussion
For those suffering from symptoms of PD despite optimal medical therapy, DBS implantation can improve quality of life by reducing tremors, rigidity, stiffness, bradykinesia, and dystonia. In addition, it can substantially reduce the doses of antiparkinsonian medications. DBS technique consists of stereotactic placement of microelectrodes in the thalamus, internal globus pallidus or STN areas, and an implantation of a battery-operated IPG device connected to the microelectrodes via the extension lead. The device delivers electrical stimulation to the specific areas of the brain that control movements and possibly acts by (1) jamming of the neuronal message transmitted through the stimulated structure; (2) desynchronizing abnormal oscillations; (3) inhibiting neuronal firing; or (4) inhibiting the production/release of certain neurotransmitters and hormones.1, 2 Stimulation from the IPG is easily adjustable by “programming,” if there is any change in patient’s symptoms.
Patient selection is important because a successful surgical outcome depends on the presence of a dopaminergic responsive system. Nondopamine responsive cases or patients with dementia and cognitive deficit are not ideal candidates for DBS implantation. The clinical relevance of intensive chest physiotherapy lies in the fact that respiratory dysfunction can occur in patients with PD, from aberrant control of respiratory muscles, excessive secretions, decreased chest wall compliance (rigidity), or upper airway obstruction (involuntary movements of glottis/supraglottic structures). Possible drug interactions include medications with extrapyramidal effects such as metoclopramide, butyrophenones, haloperidol, fluphenazines, phenothiazines, and selective serotonin reuptake inhibitors.
During cardiac surgery, use of monopolar cautery is helpful to achieve effective hemostasis from sternotomy and intrathoracic sites. It can produce electromagnetic interference with the neurotransmitter, however. Severe neurologic damage resulting from interaction of electrocautery with DBS system is reported in the literature.3 The use of bipolar cautery in short, infrequent bursts with a minimum energy setting is recommended with keeping the cautery pad as far as possible away from the neurotransmitter. Another option for dissection during cardiac surgery is the ultrasonic scalpel, which does not cause transfer of electrical energy to the tissues and is devoid of harmful effects.4 Similar to cardiac pacemaker, the DBS device requires use of magnet to reduce electromagnetic interference. Because the device is placed in close proximity to the heart, it may be damaged during cardioversion or internal defibrillation. If defibrillation is required, lowest clinically appropriate energy must be used. Better myocardial protection by administering well-timed antegrade and retrograde cardioplegia, prophylactic antiarrhythmic agents such as lignocaine/magnesium, may be helpful in restoring sinus rhythm spontaneously. Bioprosthetic valve is preferred over metallic valve, which helps in avoiding long-term anticoagulant therapy and the risk of hemorrhagic stroke. Despite considerable controversy regarding anticoagulation for the first 3 months after bioprosthetic valve replacement, addition of warfarin (Coumadin) to aspirin is recommended.5 Patients with implanted brain pacemaker can undergo surgery under cardiopulmonary bypass after 6 months without any risk of hemorrhagic complications.6
Patients with brain pacemaker may need a magnetic resonance imaging (MRI) scan to diagnose the cause of various common diseases associated with old age. Although all modern DBS systems are designed so that an MRI full-body scan is possible with proper safeguards, one should limit MRI exposure and read the specific conditions carefully. Other electronic devices that may interfere with DBS system include (1) cardiac pacemaker and implantable cardioverter defibrillator (interrogation of both devices and DBS required before and after surgery), (2) electrocardiographic (ECG) artifacts from direct effect of DBS or from severe tremors after deactivation of DBS, and (3) heating of DBS electrodes and risk of brain damage with short-wave diathermy (contraindicated).7 The devices with which no interference is reported include phacoemulsification, peripheral nerve stimulator, and electroconvulsive therapy. Device-specific manufacturer’s recommendations should always be followed.8
In conclusion, with increasing use of DBS system, special care is required in such patients because of potential risk of cerebral hemorrhage with anticoagulant therapy and/or heating of DBS electrodes, and possible interference with monitoring devices, imaging modalities, or therapeutic devices. The anesthesiologist plays an important role to ensure a safe and uneventful intraoperative environment for patients with an implanted DBS device. Relevant issues include identifying the type of device, interrogation of DBS device by a trained physician, turning off the device intraoperatively, implementing precautions when using electrosurgical equipment, and checking the device postoperatively.9
Conflict of Interest
None.
References
- Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84(2):203-214.
- [Google Scholar]
- Subthalamic high frequency stimulation resets subthalamic firing and reduces abnormal oscillations. Brain. 2005;128:2372-2382. Pt 10
- [Google Scholar]
- DBS and diathermy interaction induces severe CNS damage. Neurology. 2001;56(10):1384-1386.
- [Google Scholar]
- Use of an ultrasonic scalpel in the open-heart reoperation of a patient with pacemaker. Eur J Cardiothorac Surg. 2002;21(4):761-762.
- [Google Scholar]
- Antithrombotic therapy after bioprosthetic aortic valve replacement: ACTION Registry survey results. Eur J Cardiothorac Surg. 2008;33(4):531-536.
- [Google Scholar]
- Scheduled cardiothoracic surgery and Parkinson’s disease: how to deal with deep-brain stimulation. J Cardiothorac Vasc Anesth. 2006;20(5):707-708.
- [Google Scholar]
- Anaesthesia for deep brain stimulation and in patients with implanted neurostimulator devices. Br J Anaesth. 2009;103(2):152-165.
- [Google Scholar]
- Parkinson’s disease patient preference and experience with various methods of DBS lead placement. Parkinsonism Relat Disord. 2017;41:25-30.
- [Google Scholar]
- Anesthesia considerations for patients with an implanted deep brain stimulator undergoing surgery: a review and update. Can J Anaesth. 2017;64(3):308-319.
- [Google Scholar]






