Translate this page into:
Technical Aspects of Mitral Valve Replacement: A Guide for Beginners
*Corresponding author: Amitabh Satsangi, Assistant Professor, Department of Cardiothoracic and Vascular Surgery, New Delhi, Delhi, India. Amoeba418@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Satsangi A, Murtaza SM. Technical Aspects of Mitral Valve Replacement: A Guide for Beginners. J Card Crit Care TSS. 2024;8:57-61. doi: 10.25259/JCCC_49_2023
Abstract
Mitral valve replacement is a surgical procedure to replace a damaged or diseased mitral valve in the heart. There are different techniques for mitral valve replacement, including open-heart surgery and minimally invasive heart surgery. Here, we describe detailed steps of mitral valve replacement with a mechanical bileaflet valve which can be used by early career surgeons as a reference to perform safe cardiac surgery.
Keywords
Techniques
Mechanical valve
Mitral valve replacement
INTRODUCTION
Rheumatic valvular heart disease is the most common cause of mitral valve disease in India, specifically mitral stenosis.[1] Mitral stenosis presents earlier in the Indian[2] population, and at the time of presentation, these stenotic valves are damaged beyond repair, leaving mitral valve replacement as the only treatment option for the majority of patients. Thus, it is important for newly trained cardiac surgeons to be well-versed in the detailed steps of mitral valve replacement. There are many techniques for performing mitral valve replacement.[3-5] Here, we describe detailed techniques for performing mitral valve replacement with a mechanical bileaflet valve.
TECHNICAL ASPECTS
Midline sternotomy
After positioning the patient with a slight neck extension for exposure of the supraclavicular notch, the patient is painted and draped. The midline sternal incision is marked with a supraclavicular notch and the xiphisternum is in line. A skin incision is given, and then, using diathermy, the subcutaneous fat is split until the sternum is reached. At the xiphisternum, the anterior rectus sheath is divided, creating a space. At this point, a vein near the xiphisternum is identified and cauterized. The supraclavicular notch is approached, and the interclavicular ligament is identified and divided. The midline sternotomy is done. Hemostasis is achieved on the outer and inner tables of the cut sternal bone.
COMMENCEMENT OF CARDIOPULMONARY BYPASS (CBP)
Thymus tissue is split, and an inverted T-shaped vertical pericardiotomy is done. Pericardial stays are applied. Two for the aorta, one for the superior vena cava (SVC), one for the right atrium, one for the inferior vena cava (IVC), and one near the pulmonary artery. A double aortic purse string is taken, and heparin is given. After 3 min, aortic cannulation is done. Then, the SVC U stitch is taken, and SVC cannulation is done. When the activated clotting time is 3 times the baseline or more than 480, the patient is put on partial bypass. The IVC purse string is taken, and then, the perfusionist is instructed to fill the heart, and IVC cannulation is done. The patient is now on full bypass. The perfusionist is instructed to cool the patient to 32°. The cardioplegia purse string is taken, and the cardioplegia cannula is placed. Cardioplegia is flushed, and the line is de-aired and connected to the cardioplegia cannula. The interatrial groove is developed to delineate the left atrium either by scissors or using diathermy.
AORTIC CROSS-CLAMP AND MYOCARDIAL PRESERVATION
The patient is ready to apply the aortic cross-clamp. Before applying the cross-clamp, it is necessary to ensure the presence of an ice-cold solution for topical cooling and for the nursing officer to be ready with a scalpel to immediately vent the left ventricle through the left atrium just after applying the aortic cross-clamp. The perfusionist is instructed to decrease the flows, and the aortic cross-clamp is applied. The left atrium is opened, and a vent is placed in the left ventricle through the mitral valve. During venting the left ventricle through the mitral valve, it is important to note that sometimes, the left ventricle is not vented even though a vent is placed, and it is important to frustrate the mitral valve using a Semb’s ligature carrier to achieve proper left ventricle venting. Then, the cardioplegia is started and given over 5 min, along with topical cooling with ice-cold water. When the cardioplegia has been given, the left atrium incision is increased using Pott scissors. A left atrial retractor is placed. The first step is to look into the left atrial appendage for any clots. Once the left atrial appendage is cleared of clots, the mitral valve is visualized.
MITRAL VALVE EXCISION
The anterior leaflet of the mitral valve is identified, and a valve hook is used to pull the anterior mitral leaflet (AML) toward the surgeon for better exposure of the mitral valve [Figure 1a]. An incision is made at the annulus and AML junction, 2 mm away from the annulus, using an 11-no. The blade on a scalpel at the 12 o’clock position is shown in Figure 1b. After the incision, the AML is held using right-angle forceps. The incision is further extended to the 9 o’clock and 3 o’clock positions. Two stays using 2-0 polyesters are put in the 3 o’clock and 9 o’clock positions [Figure 1c]. These stays are very important to improve the exposure of the mitral valve and aid in passing mitral valve sutures. After putting on sutures, the AML is divided into two parts, and the subvalvular apparatus is visualized and scrutinized to look for chordal preservation. If feasible, then small buttons (1 × 1 cm) are made for anterior chordal preservation. These buttons are then sutured to the anterior and posterior commissures of the mitral valve annulus. The posterior leaflet is then examined; if the leaflet is thin and pliable, a little shaving is done, and posterior chordal preservation is tried. If there is excessive calcification, then the posterior leaflet is trimmed as much as possible. After the native valve has been excised, a thorough wash is given using cold saline. The valve is sized using an appropriate sizer [Figure 1d]. Four operating towels are placed around the chest retractor.
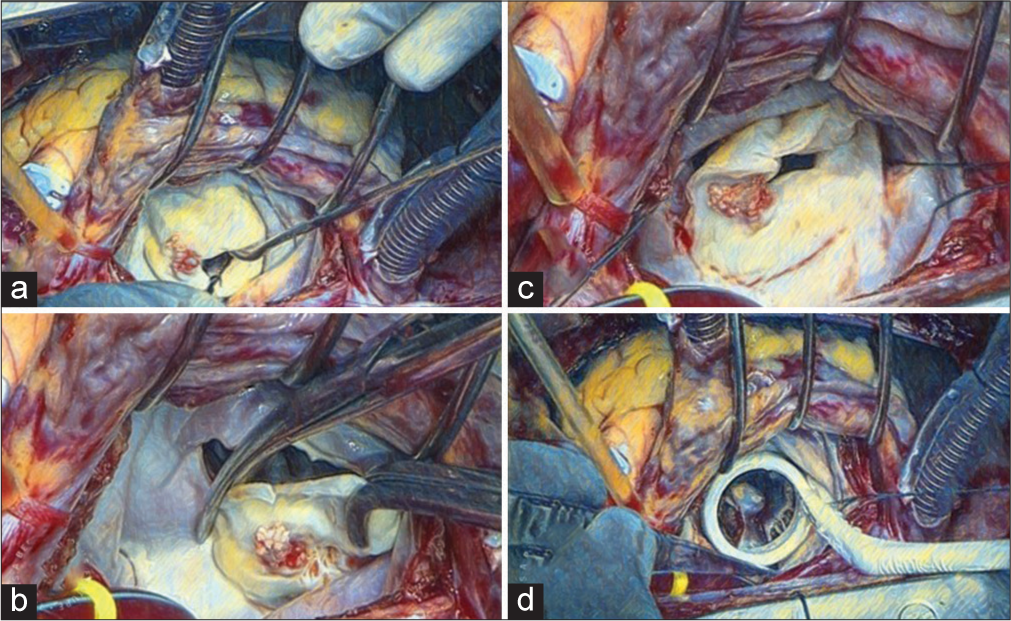
- (a) Mitral valve pulled toward the surgeon using valve hook for better visualization of mitral valve. (b) Anterior mitral leaflet being excised. (c) Commissural stays placed at anterolateral and posteromedial commissures. (d) Mitral valve being sized.
CHORDAL PRESERVATION TECHNIQUES
Various methods of chordal preservation have been described: [6]
Posterior mitral leaflet preservation technique of Lillehei
David’s technique
Feike’s technique
Miki’s technique
Khonsari
Rose and Oz.
Advantages
Preserves left ventricular (LV) geometry
Preserves LV function
Reduces operative mortality
Improves early and long-term survival
Reduces risk of ventricular rupture
Improves right ventricular function
Reduces risk of left ventricular outflow tract obstruction (LVOTO).
MITRAL VALVE REPLACEMENT
The valve is taken, and the valve is held by the second assistant, with the valve holder facing the operating surgeon and the valve placed in an anti-anatomical position. All the bites are taken as backhand for the anterior annulus, and the bites are first taken at the sewing ring, and then, they are passed through the annulus from the left ventricle to the left atrium [Figure 2a]. Polyester 2’0 non-pledged sutures are used, and the first suture is passed at 12 o’clock of the sewing ring of the valve and then at 12 o’clock at the valve annulus. While taking the suture at the annulus, the 9 o’clock stay suture is held and kept taut to provide better visualization of the annulus. After the suture is passed, the suture is given to the second assistant to hold to provide traction and to keep the sutures in the opposite direction of the next suture to be taken. The next bite is taken toward the 11 o’clock position after passing through the sewing ring of the valve [Figure 2b]. The sutures in this quadrant are completed similarly until the 9 o’clock position at the annulus. Then, sutures, usually three pairs (six needles), are collected in a snap, and the needles are cut. Then, the second quadrant is started with the suture first being passed near the initial 12 o’clock suture and then to the corresponding place at the annulus. To improve the annulus exposure, the 3 o’clock stay is held taut. Now, the sutures are advanced to the 3 o’clock position at the annulus, and then, the sutures are held by a snap and needles are cut [Figure 2c]. Now, the valve is shifted toward the first assistant, although it is still being held by the second assistant. The next suture is now taken forehand and passed at the 6 o’clock position at the sewing ring and then the posterior annulus at the 6 o’clock position. At this point, it is important to imbricate the posterior leaflet that is left for posterior chordal preservation. Now, with the suture, we move toward the 9 o’clock position at the annulus from the 6 o’clock position. After the quadrant is completed, the sutures are collected and held by a snap, and the needles are cut. Similarly, in the last quadrant, from 6 o’clock to 3 o’clock, the sutures are passed. After all the sutures are done, the stay sutures are removed. The valve and holder are held by the operating surgeon in the right hand, and the snaps and sutures are held by the surgeon and the assistants. The valve is meticulously wetted and then parachuted toward the annulus [Figure 2d]. After the valve has come down, the holding apparatus is detached from the valve. Now, the valve along with the suture is checked to see if any suture is loose or stuck. After ensuring all the sutures are properly placed, the sutures are tied. First, the 12 o’clock position suture is tied. While tying the suture, both arms are swung to check if they belong to the same suture. This is a very important step and should be performed before tying each suture. Then, the next at 6 o’clock suture is tied. Snaps are placed at 12 o’clock and 6 o’clock tied sutures. Now, we start tying the sutures in a clockwise manner, where each quadrant is tied and a snap is placed [Figure 3a]. A minimum of six knots have to be tied, with the knot being crossed. After all the knots are tied, the knots are cut in a clockwise position and pulled before cutting to check if the knots are tight. At 12 o’clock and 6 o’clock, sutures are not cut. Now, the valve is checked with a valve tester, and we look for any loose threads. A nerve hook is used for any paravalvular leaks, and the valve is rotated if required to the anti-anatomical position.
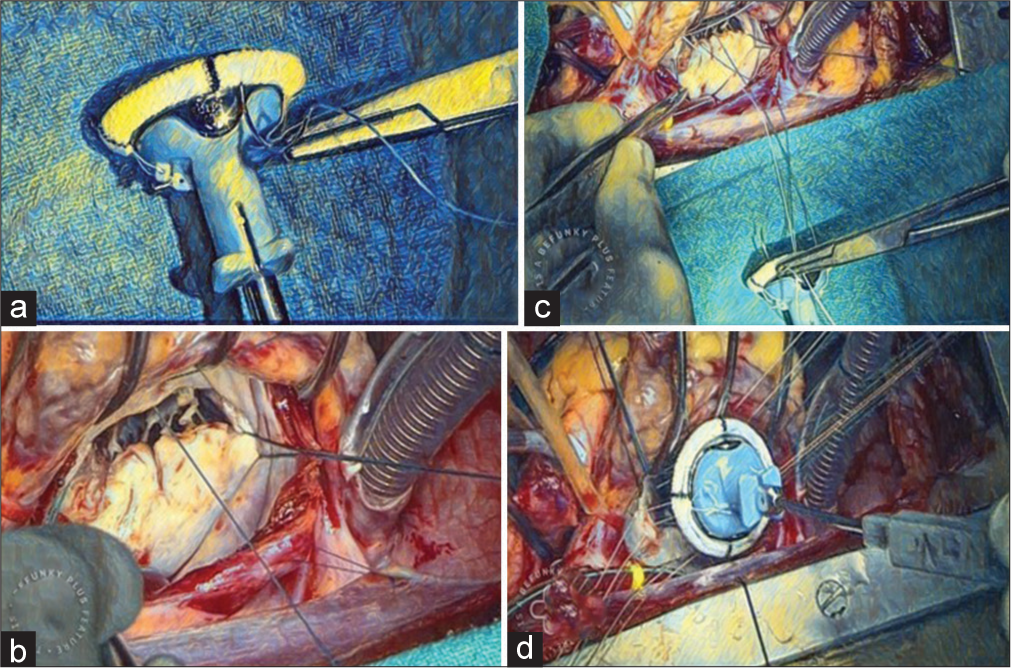
- (a) Suture being placed at 12 o’clock position at the sewing ring. (b) Annulus suture taken at 12 o’clock position at mitral annulus. (c) Mechanical valve to mitral annulus suturing using interrupted suture technique. (d) Mechanical mitral valve being parachuted.
REWARMING
The perfusionist is instructed to start rewarming the patient. The patient is rewarmed to about 35°C using the TCM (heating and cooling system) and forced air patient warming system. During the rewarming phase, only warm saline is used for any purpose. Adequate rewarming is necessary for getting good contractions and heart rate.
THE DE-AIRING OF THE HEART
A Foley catheter is placed to frustrate the mechanical valve, with the Foleys placed between the two leaflets of the bileaflet mechanical valve [Figure 3b]. If the left atrial appendage has to be ligated, it should be ligated on the clamp as it is easier to ligate on the clamp. After the Foleys are inflated, the root vent is connected to the cardioplegia cannula, the head end is lowered down, the perfusionist is advised to lower down the flows in the aortic cannula, the aortic root is pinched, and the aortic cross-clamp is released. Minimal inotropes are started during this phase, preferentially dobutamine and glyceryl trinitrate (NTG) infusions. The inotropes help in providing support to the heart while coming off bypass. The heart is aired in a retrograde and antegrade manner. The left atrium closure is done using two 3’0 polypropylene 26-mm needle sutures. The first layer is done backhand and advanced from below to upward. Similarly, the next half of the first layer is done from above downward. The Foleys are crossed, with attention being given not to take the Foleys into the suture. Once the ventricle starts beating, the perfusionist is advised to fill the heart, and the anesthetist is advised to bag ventilate the patient to de-air the left side of the heart. If transesophageal echo is present, it is used to see adequate de-airing of the left heart. The Foleys are now removed. After removing the Foley, forceps are placed into the left atrium through the suture line, and the heart is further de-aired by filling the heart and bag ventilation under trans-esophagel echocardiography (TEE) guidance. After adequate de-airing of the left heart, the forceps are removed and the left atrium suture is tied, holding the anesthetic bag ventilation in place, and the sutures are tied. Now, the second layer of left atrial closure is done.
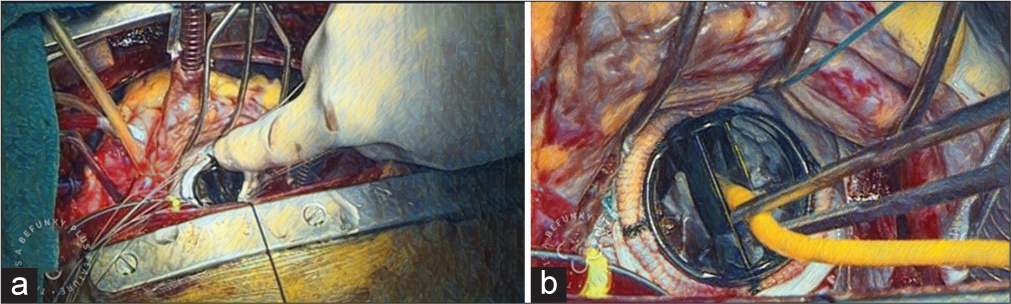
- (a) Tying of mechanical mitral valve to the mitral valve annulus. (b) Frustration of the mechanical valve with Foley catheter.
SEPARATION FROM CARDIOPULMONARY BYPASS
Ideally, four pacing wires are placed: Two ventricles and two atrial. After placing ventricle wires, the patient is put on partial bypass by removing the IVC cannula and reinforcing the IVC cannulation site with a 4’0 polypropylene suture. Atrial pacing wires are placed. After placement of atrial pacing wires, the patient is put on full ventilation, hemoglobin, electrolytes, and acid-base balance is checked, and if within the acceptable range, the patient is separated from the CPB machine. When successfully off the CPB machine, TEE is done and checked for mechanical valve leaflet movement, any paravalvular leak, aortic valve function, tricuspid valve function, and the presence of any air in the left heart. Once everything seems fine, then venous decannulation followed by aortic root vent is removed (after 5 min of coming off bypass), and reinforcements are taken first at the aortic root vent site with a 4’0 polypropylene suture, and the SVC cannulation site is kept snugged. Protamine is infused, and the aortic cannula is kept until 50% of the protamine has been administered. SVC reinforcement is done using 4’0 polypropylene. Aortic decannulation is performed, and reinforcement is done with a 4’0 polypropylene suture. After hemostasis is achieved, pericardial drains are placed and tied, and pacing wire fixation to the skin is done. The pericardium is completely closed. Hemostasis is further achieved at the sternal level, and sternal closure is done either using steel wire or the number 5 Ethibond suture. A total of four figures of eight sutures are placed, and after placing the sutures, needle holes are checked for bleeding. When satisfactory, the sternum is closed, and routine closure of subcutaneous tissue and skin is done.
DISCUSSION
Mitral valve replacement is a surgical procedure that can be performed using different techniques, depending on the patient’s condition and the surgeon’s preference. Mitral valve replacement is a complex surgical procedure that requires careful consideration of the patient’s condition and the best technique to use. Open-heart surgery is the most common technique, but minimally invasive surgery and robot-assisted surgery are becoming more widely used. Each technique has its advantages and disadvantages, and the choice of technique should be based on the patient’s individual needs and the surgeon’s expertise.[7,8] The surgical replacement of a stenotic or insufficient mitral valve is based on the premise that the prosthesis type chosen will have a beneficial impact on cardiac function and quality of life within the context of perioperative risks and long-term prosthesis complications. Minimal access, endoscopic, and robotic approaches are becoming increasingly established as excellent surgical alternatives. However, the current device costs in an unfavorable economic climate, in addition to significant learning curves in an era of uncompromising quality control, limit its application to centers of excellence with established experience. There are many techniques for performing mitral valve replacement, and the technical aspects differ from one surgeon to another. The mitral valve itself can be approached through the left atrium, right atrium, bi-atrial approach, approach through the roof of the left atrium, trans-LV, and transaortic root.[9] Here, we have described the standard trans left atrial approach and the use of the interrupted suturing technique. In our manuscript, we have tried to summarize the most technical and practical aspects of mitral valve replacement that can be reviewed by surgeons before performing mitral valve replacement. Everting or non-everting sutures should be placed into the annulus with attention to avoid injury to the non-coronary aortic valve leaflet, circumflex coronary artery, and atrioventricular conduction tissue located in close proximity. It is generally recommended that mechanical prostheses should be oriented in an anti-anatomic fashion and bioprosthetic strut locations should be oriented such that contact with the ventricular wall and impingement on the LV outflow tract is avoided. LV rupture occurs in 1% of procedures and can occur at the level of the annulus, papillary muscles, or mid-ventricular zones. It is associated with aggressive decalcification and endocardial disruption that results in the inter myocardial fiber dissection of blood, with a subsequently reported mortality of 50%.[10] Immediate recognition and replacement of the valve with dissection tract incorporation are required.
CONCLUSION
Safe mitral valve replacement using a mechanical valve can be performed if the technical steps are meticulously followed.
Ethical Approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Incidence and Patterns of Valvular Heart Disease in a Tertiary Care High-volume Cardiac Center: A Single Center Experience. Indian Heart J. 2014;66:320-6.
- [CrossRef] [PubMed] [Google Scholar]
- Mitral Valve Replacement with Preservation of Chordae Tendineae and Papillary Muscles. Ann Thorac Surg. 1988;45:28-34.
- [CrossRef] [PubMed] [Google Scholar]
- Mitral Valve Replacement-current and Future Perspectives. Open J Cardiovasc Surg. 2017;9:1179065217719023.
- [CrossRef] [PubMed] [Google Scholar]
- Mitral Valve Replacement: Technique to Preserve the Subvalvular Apparatus. Ann Thorac Surg. 1995;59:1027-9.
- [CrossRef] [PubMed] [Google Scholar]
- Chordal Preservation in Mitral Valve Replacement. Opteckcs. 1998;3:130-3.
- [CrossRef] [Google Scholar]
- Mitral Valve Replacement for Mitral Regurgitation with and without Preservation of Chordae Tendineae. J Thorac Cardiovasc Surg. 1984;88:718-25.
- [CrossRef] [PubMed] [Google Scholar]
- Mitral Valve Repair: Robotic and Other Minimally Invasive Approaches. Prog Cardiovasc Dis. 2017;60:394-404.
- [CrossRef] [PubMed] [Google Scholar]
- Extended Vertical Transatrial Septal Approach to the Mitral Valve. Ann Thorac Surg. 1991;52:1058-60.
- [CrossRef] [PubMed] [Google Scholar]
- Rupture of Left Ventricle Following Mitral Valve Replacement. Ann Thorac Surg. 1988;46:590-7.
- [CrossRef] [PubMed] [Google Scholar]






