Translate this page into:
ECMO and Ventricular Assist Devices as a Bridge to Transplant
Poonam Malhotra Kapoor, MD, DNB, MNAMS, FIACTA (Hony), FTEE (Hony), FISCU (Hony) Department of Cardiac Anaesthesia and Critical Care, AIIMS New Delhi 110029 India drpoonamaiims@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Heart Failure Management
Many cardiac diseases, like coronary artery disease and some viral cardiomyopathy over a period of time, give rise to the condition called heart failure (HF). More than 2 million Indians suffer from HF today. The first line of management includes lifestyle modifications, as well as medical therapy. The latter uses drugs such as beta blockers, angiotensin-converting enzyme inhibitors, angiotensin receptors/blockers aldosterone antagonists, diuretics, digoxin, and many others keep getting added with more research and research and development today. Salt and fluid restriction too plays a pivotal role. Patients with a wide QRS complex in their electrocardiogram and those having a moderate to severe symptoms of HF need to have a cardiac resynchronization therapy and a patient with fatal arrhythmias needs an implantable defibrillator inserted as therapy. So, use of advanced mechanical therapies is a necessity in HF patient.
Most HF Indians have class III or class IV symptoms of dyspnea at rest of some duration or a recent origin. In this scenario of advanced mechanical support in HF patient, the two definitive therapies of nearly 6,000 in number and still on the increase are the use of ventricular assist devices (VAD) and heart transplant, which have met with some success (Box 1). This editorial briefly revises extracorporeal membrane oxygenation (ECMO) as a bridge to transplant (Figs. 1 and 2).
|
• Integrated ECMO circuitry was established along with CPB circuit and primed with blood • Advantages of integrated circuit of them: 1. No time is lost from decision to initiation of ECMO 2. Early Initiation may prevent end organ damage 3. With a use of integrated ECMO, surgical asepsis is maintained 4. The procedure is cost-effective 5. Increasing survival in these critically ill patients |
Abbreviations: CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation.
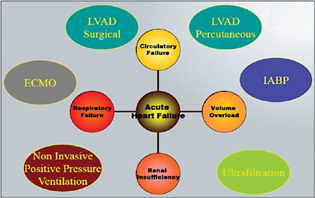
- Mechanical assist devices in heart failure patients. (Adapted with permission and courtesy to Jaypee Publishers, New Delhi, Manual of ECMO in ICU 2018, 2nd ed.) ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device.
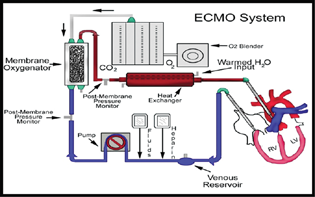
- The typical extracorporeal membrane oxygenation (ECMO) circuit.
Extracorporeal Membrane Oxygenation
ECMO is used in cardiac or pulmonary failure in moribund patients, wherein supraoptimal medical therapy does not work at all (Figs. 3A and B). Venovenous ECMO provides adequate oxygenation and carbon dioxide removal in isolated refractory respiratory failure, while venoarterial (VA) ECMO when support is required for cardiac and/or respiratory failure.1
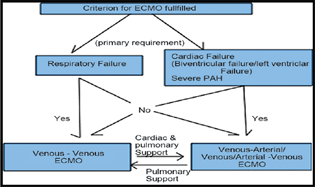
- Choice of extracorporeal membrane oxygenation (ECMO) in different clinical scenarios.
Understanding the CHOICE of ECMO
Patient on VA ECMO Severe Systolic and Diastolic MR
ECMO Complications
While on ECMO one needs to worry of its complications as well, which may vary from sepsis to thrombosis. Echocardiography (ECHO) aids in helping delineate ECMO complications. Multiple views of ECHO (both two- and three-dimensional) help in ECMO success (Figs. 4A and B).2, 3
ECMO Complications—Flow Obstruction
Obstruction of the inferior vena cava has been seen on a transesophageal echocardiography in the lower hepatic view (Fig. 5).
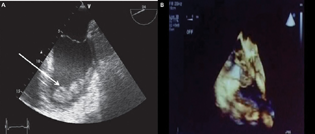
- Intraventricular thrombosis is a well-known complication of myocardial infraction. Extracorporeal membrane oxygenation (ECMO) and left ventricular akinesis can increase this risk (A) seen in an ECHO picture as thrombus or cast embolus or cause flow obstruction to superior vena cava/inferior vena cava (B). Complications of ECMO may vary from and result from rupture or dysfunction of any ECMO circuit component.
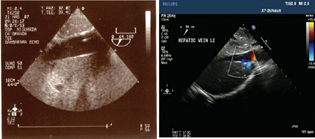
- Obstruction of the inferior vena cava.
The use of integrated ECMO circuit benefits the patients well as shown in Box 1.4
Whenever using ECMO as a bridge to transplant or a bridge to recovery or a bridge to VAD or a bridge to nowhere important to remember that ECMO is a transient and not the definitive therapy, thus it is used as a bridge. ECMO helps as a therapeutic option while awaiting an organ for transplant or a VAD for definitive therapy.
When weaning from ECMO thus though no definitive weaning guidelines exist we clinical judgement, hemodynamic parameters and ECHO variables are useful. Monitoring on ECMO is important (Box 2) and knowledge of ECMO advantages in HF patients is very useful (Box 3 and 4).
|
• ScVO2 >70% • MAP >60 mm Hg (in case of full support with nonpulsatile flow) • MAP >70 mm Hg (in case of partial support with pulsatile flow) • Avoid full flow and give partial flow about 2/3 through ECMO • Maintain oxygen saturation of 90% or PaO2 of >50 mm Hg (in young patient) and >60 (in elderly and with CAD or CVA) • Maintain coronary saturation of >70% |
Abbreviations: CAD, coronary artery disease; CVA, cardiovascular accident; ECMO, extracorporeal membrane oxygenation; MAP, mean arterial pressure; PaO2, partial oxygen pressure; ScVO2, central venous oxygen saturation.
|
• ECMO can be inserted at the bedside • It is relatively cheap (as compared to the implantable LVAD) • Gives the treating physicians some time to wait for recovery or a more stable condition • Portable, can be inserted in Cath lab using 17-21Fr cannulas • IABP to reduce afterload • Centrifugal pump at 2–4L/min • Bridge to surgical VAD, transplant or recovery |
Abbreviations: ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device.
|
▪ Possible fields of application: Respiratory failure, such as ARDS, ALI, VILI ▪ Patient and user values: Extracorporeal CO2 removal enables protective ventilation ▪ Special feature: Support of gas exchange for up to 30 days without exchanging components with focus on low flow and CO2 removal instead of oxygenation ▪ Cannulation: Percutaneous cannulation of either jugular, subclavian, or femoral vein |
Abbreviations: ALI, acute lung injury; ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; VILI, ventilator-induced lung injury.
Future of ECMO
Applications for ECMO have expanded today, which also include percutaneous temporary left ventricular assistance and low-flow ECMO for CO2 removal. With new equipment of ECMO machinery, like oxygenators, pump and surface coatings, there is improvement in ECMO procedure, both by becoming more simple and more safe (Fig. 6).5, 6, 7
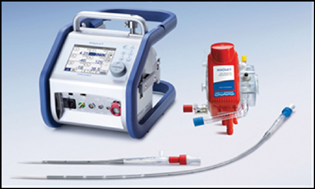
- The Cardiohelp. (Adapted with permission and courtesy to Jaypee Publishers, New Delhi, Manual of ECMO in ICU 2018, 2nd ed.)
Ventricular Assist Devices as a Bridge to Transplant
VAD is a mechanical pump that is surgically attached to one of the heart's ventricles to augment or replace native ventricular function. It can be used for the left ventricular assist device (LVAD), right VAD), or both ventricles. They are provided with external power sources that connect to the implanted pump via a percutaneous lead (driveline) that exits the body on the right abdomen. Pump output flow can be pulsatile or nonpulsatile. The need for a VAD in heart disease patients is felt even more acutely, today in 2021, than ever before as the number of patients with heart disease rises. Heart disease is the leading cause of death in the Western world. Approximately five million people in the United States have congestive heart failure.
Left Ventricular Assist Devices
LVADs are increasingly used as a bridge to transplantation. It remains unclear whether the use of pre transplant LVADs adversely affects short-term survival after cardiac transplantation (Fig. 7).
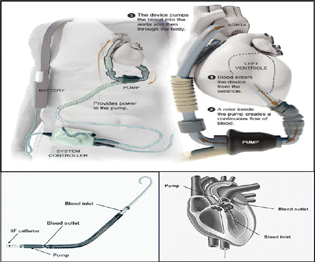
- Impella and HeartMate I, two VADS in Global use.
Components of an LVAD
The LVAD consists of a pump with controller, cannulas, and power source as explained in Box 8. Each component has its own sets on complications (Box 4 5 6 7 8).
|
● 250,000 are in the most advanced stage of CHF ● ∼500,000 new cases each year ● ∼50,000 deaths each year ● Only effective treatment for end-stage CHF is heart transplant |
Abbreviations: CHF, congestive heart failure; VAD, ventricular assist device.
|
➢ Bridge to transplant (BTT) • Most common • Allow rehab from severe CHF while awaiting donor |
|
➢ “Destination” therapy (DT) • Permanent device, instead of transplant • Currently only in transplant-ineligible patients |
|
➢ Bridge to recovery (BTR) • Unload heart, allow “reverse remodeling” • Can be short- or long-term |
|
➢ Bridge to candidacy (BTC)/ Bridge to decision (BTD) • When eligibility unclear at implant • Not true “indication” but true for many pts |
Abbreviations: CHF, congestive heart failure; VAD, ventricular assist device.
|
• Bridge to transplant (BTT) – Intention is for patient to go to transplant – May prevent death on the wait list |
|
• Destination therapy (DT) – Heart Mate XVE and Heart Mate II are the only LVADs approved for this purpose – Intention is for LVAD therapy to be indefinite |
|
• Bridge to recovery – Usually difficult to predict with certainty |
|
• Bridge to decision – When doubt remains in the actual therapy or while awaiting an organ |
Abbreviation: LVAD, left ventricular assist device.
|
• Cannula • Pump – Internal • Controller (computer) • Power source – Always external – Batteries – Wall unit |
Abbreviation: LVAD, left ventricular assist device.
LVAD versus Transplant
Malignancy is typically a contraindication for heart transplantation, but LVAD may be a consideration. There are no strict age limit for LVAD and no immunosuppressive therapy for LVAD. If there are psychosocial factors that make the patient ineligible for transplant, it is unlikely that the patient will be an LVAD candidate.
Summary
Advances in ECMO and VAD technology have made durable mechanical assist therapy available for select severely symptomatic HF patients. These pumps are surgically implanted at some tertiary care centers. Demonstrate mortality and quality of Life benefit patients must be undergo selection similar to heart transplant evaluation. Devices may become an important component in the management of acute HF. Although devices improve hemodynamic and metabolic parameters, there is currently no proven mortality benefit. These devices are not without complication; therefore selection of patient and device is key, requiring an integrated stepwise approach. ECMO in ADHF serves as a bridge to LVAD.
Conflict of Interest
None declared.
References
- Extracorporeal membrane oxygenation–an anaesthesiologist's perspective–Part II: clinical and technical consideration. Ann Card Anaesth. 2012;15(1):69-82.
- [Google Scholar]
- Introduction to Extracorporeal Membrane Oxygenation.Manual of ECMO in ICU. (2nd ed.). New Delhi: Jaypee Publishers; 2018. p. :3-6.
- [Google Scholar]
- Echocardiography in extracorporeal membrane oxygenation. Ann Card Anaesth. 2017;20:S1-S3. (Supplement):
- [Google Scholar]
- Use of integrated extracorporeal membrane oxygenator in anomalous left coronary artery to pulmonary artery: better survival benefit. Ann Card Anaesth. 2011;14(3):240-242.
- [Google Scholar]
- Role of Extracorporeal Membrane Oxygenation for Acute Decompensated Heart Failure. Manual of ECMO in ICU. (2nd ed.). New Delhi: Jaypee Publishers; 2018. p. :205-9.
- [Google Scholar]
- Low flow venoarterial ECMO support management in postcardiac surgery patient. J Cardiac Criti Care TSS. 2021;5(2):103-107.
- [Google Scholar]
- Cardio Help: The Future of Extracorporeal Membrane Oxygenation. Manual of Extracorporeal Membrane Oxygenation (ECMO) in the ICU. (1st ed.). New Delhi: Jaypee Publishers; 2014. p. :243-248.
- [Google Scholar]





