Translate this page into:
Viscoelastic Testing on Venoarterial Extracorporeal Membrane Oxygenation: Need or Greed?
*Corresponding author: Poonam Malhotra Kapoor, Professor, Department of Cardiac Anaesthesia and Critical Care, CNC, All India Institute of Medical Sciences, New Delhi, Delhi, India. drpoonamaiims@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Malhotra Kapoor P, Garg S, Prakash M, Mujahid OM, Thiruselvan T. Viscoelastic Testing on Venoarterial Extracorporeal Membrane Oxygenation: Need or Greed?. J Card Crit Care TSS 2023;7:118-28.
Abstract
Extracorporeal life support (ECLS) is a life support modality that is being used in life-threatening cardiac and/or respiratory failure; in neonates, children, and adults. The risk of bleeding and other coagulation-related issues is unavoidable. Hence, while conducting ECLS, a patient-tailored approach is better than the generalized approach for bleeding management. There are no established guidelines for heparin use and its monitoring during ECLS in a bleeding patient on VA ECMO. Likewise, heparin and its adjuncts, though mentioned in the literature, have no consensus on what exact steps to follow in an adverse condition. Having a protocol for anticoagulation and its monitoring is of paramount importance in any center practicing ECLS. This review aims to seek the incidence of bleeding and thrombosis while on ECMO with the use of routine anticoagulant heparin and justify the need for the use of viscoelastic tests on VA ECMO.
Keywords
Thrombosis
Heparin
Clot
Anticoagulation
Extracorporeal membrane oxygenation
INTRODUCTION
The term extracorporeal is self-explanatory (extra-outside; corpus-body). It is the process of carrying the blood outside the body and enriching it by oxygen and carbon dioxide removal (and also substrate filtration, etc.) and finally returning it to the patient. Extracorporeal life support (ECLS), thus, is taking over the patients blood on an artificial endothelium of the ECMO machine. Here the role of the heart is taken over by the centrifugal pump and the lung by an oxygenator.[1-3] Extracorporeal membrane oxygenation (ECMO) is a life support modality that is increasingly being sought after as a rescue measure during life-threatening cardiac and/or respiratory failure; in neonates, children, and adults alike particularly post-COVID-19 pandemic.[4]
The continuous contact of the patient’s blood with an exogenous surface results in a shift of the normal hemostatic balance toward a pro-coagulant state and there is thus a risk of thrombosis at all times[5] [Figure 1]. Administration of antithrombotic therapy is therefore essential to suppress this hemostatic activation and prevent thrombosis in both the patient and the circuit.[5] Ideal anticoagulation on ECMO is still an unsolved issue in the conduct of ECLS. An ideal anticoagulant (AC) should achieve a desirable amount of anticoagulation along with reduced or no bleeding risks. Heparin due to its ease of administration, easy availability, and easy to neutralize with protamine has been the gold standard so far!
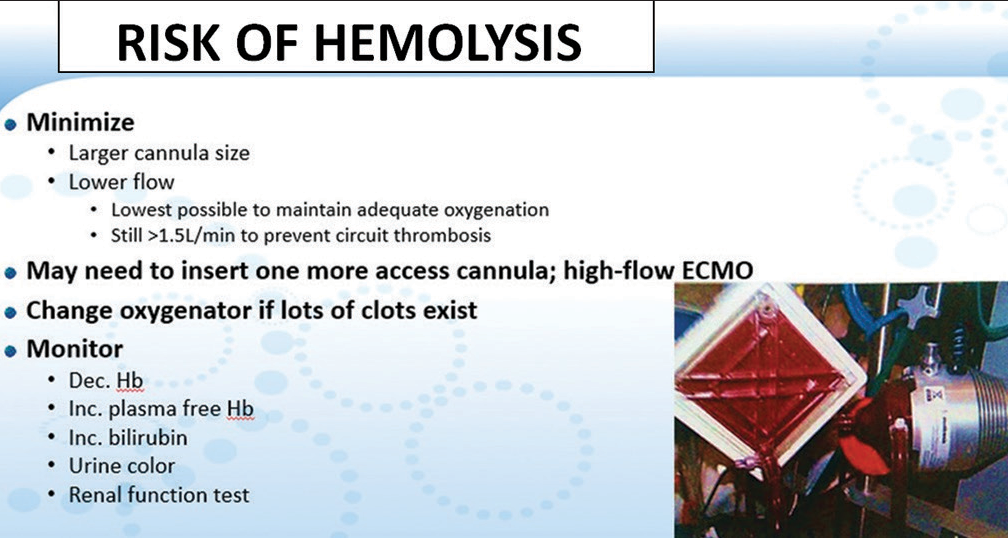
- Risk of hemolysis seen on the ECMO oxygenator.
Contact of blood and blood products with the circuit tubings and other foreign surfaces makes them vulnerable to coagulopathy. Sometimes, the dilutional effect of ECLS causes reduced factors which tend to cause more bleeding. Hence, the tight balance between bleeding and thrombosis exists when normal physiological intact endothelium is disrupted as in VA ECMO.[6]
MATERIAL AND METHODS
This narrative review was carried out by researchers using the recommendations made by the Cochrane Collaboration and following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [Figure 2]. This study was approved by the Ethics Committee of All India Institute of Medical Sciences (AIIMS) (Trial no IECPG-22/23.01.2019) for monitoring coagulopathy of up to 70 patients on venoarterial (VA) ECMO. The protocol is also registered in PROSPERO with ID 413439. As ECMO is carried out routinely on our cardiac patients, for this review, no funding resources were used except our personal computers and software.
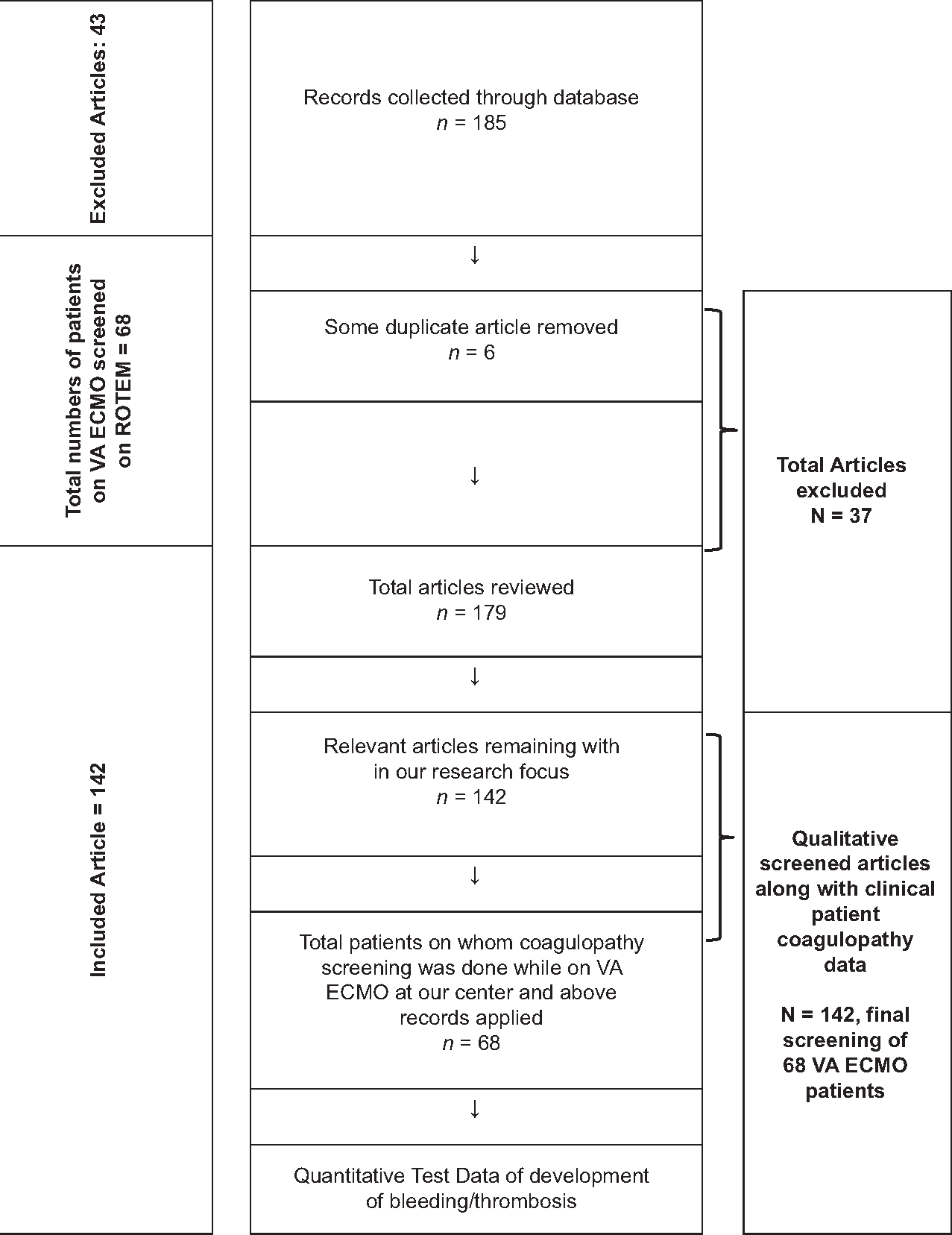
- Flow chart used for reporting items for selecting patients in this systematic review for monitoring coagulopathy on VA ECMO patients.
The primary endpoint os this research was
Bleeding beyond 200 mL over 48 hours with the ideal use of anticoagulant (AC) heparin and/or its alternative bivalirudin.
The secondary endpoint was the development of:
Thrombocytopenia and/or
Thrombosis (circuit and systemic) or both on VA ECMO circuit/patient for upto 48 hours.
The inclusion criteria for reviewing patient selection and monitoring on VA ECMO
The following criteria were included in the study:
Prospective observational study where heparin was used as the sole AC on ECMO and/or bivalirudin
Patients of both genders aged 1–60 years
Patients on VA ECMO post-cardiac surgery.
Exclusion criteria
The following criteria were excluded from the study:
Retrospective studies
Age >60 years
Non-cardiac surgery
Non heparin AC
Patients on pre-operative antiplatelet therapy.
Research Strategy (2019–2023)
A literature search was conducted in the main databases from 2002 to July 2023, using search engines such as MEDLINE, Google Scholar, PubMed, Cochrane, and EMBASE database. In addition, to reduce publication bias, the search was carried out on the individual citations of the articles found. A combination of the terms ECMO, bleeding, thrombus, clot, thrombocytopenia, and all MeSH were used as keywords.
Study selection
Two authors independently assessed the inclusion criteria in all selected studies by title and abstract. Two authors extracted the data from the studies chosen with full text, and in the case of disagreements, the issue was resolved with a third author. The data were entered into the Stata software to assess its accuracy [Figure 3]. Selective reporting is an important and notorious bias in these kinds of studies. Hence, they were taken care of vigilantly by both the chief researchers.
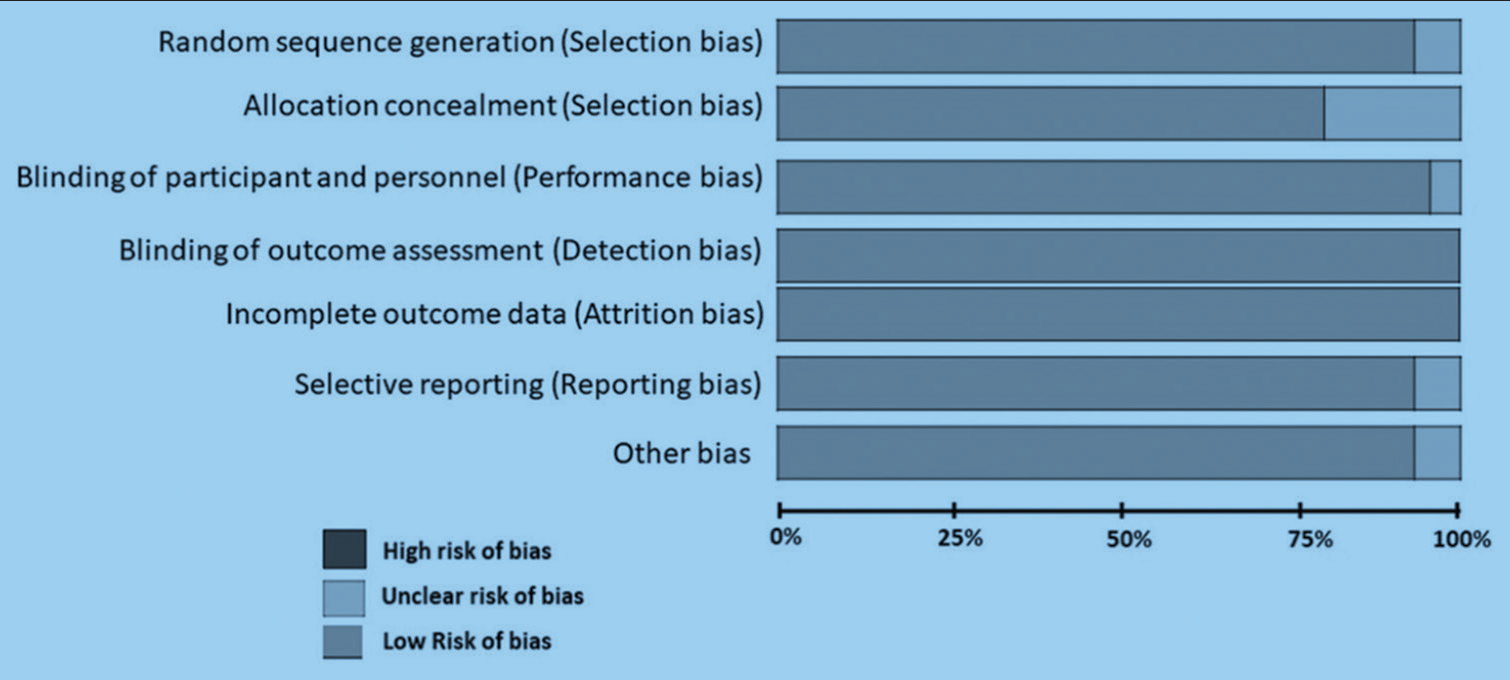
- Risk of bias assessment done on venoarterial extracorporeal membrane oxygenation (ECMO) patients, while monitoring bleeding and thrombosis, with the use of ECMO.
MONITORING ANTICOAGULATION ON ECMO
The majority of monitoring tests used for monitoring anticoagulation on ECMO are plasma-based tests. These latter tests include, activated partial thromboplastin time (aPTT) of Kaolin, and activated clotting time (ACT) both of which measure coagulation partially, and do not take into account platelet function or clot strength. The information about the platelet function for which platelet function assay-100 is to be utilized is generally not available in most ECMO centers as it is expensive. Viscoelastic tests (VET) are whole blood tests, which measure both clot strength and platelet function but are not routinely employed during ECMO. They are expensive and also require expertise in their performance and interpretation for the underlying deficiency factor causing ECMO bleeding or thrombosis [Table 1]. Besides, their availability even in 2023, in most ECMO centers of India, is scarce. Factors associated with increased risk of thrombosis are listed in Table 2.
| S. No. |
Test | Sample to be sent | Indication | Target Range | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| 1. | ACT | Whole blood | To monitor anticoagulant effect | 180–220 s |
|
|
| 2. | aPTT | Plasma | To monitor AC effect | 25–90 s or 1.5–2.5 times baseline (depending on the device, AC, and utilized method) |
|
|
| 3. | D-dimer | Whole blood or plasma | To monitor fibrin formation and fibrinolysis in the circuit and patient’s body |
0.28–1 mg/L |
|
|
| 4. | PT/INR | Plasma | To assess coagulation deficit To monitor Vitamin K antagonists |
< 1.5 for heparin-treated patients>2–3 for other ACs |
|
|
| 5. | Anti-factor Xa assay | Plasma | To monitor AC effect | 0.3–0.7 IU/mL |
|
|
| 6. | ECT | Plasma | To monitor the effect of DTIs | 300–500 s |
|
|
| 7. | Viscoelastic tests (ROTEM/ TEG) | Whole blood | To assess clotting time (AC effect), thrombocytopenia, hypofibrinogenemia, and fibrinolysis | Not established |
|
|
CRP: C-Reactive Protein, aPTT: Activated partial thromboplastin time, PT/INR: Prothrombin time/international normalized ratio, DTI: Direct thrombin inhibitors, ECT: Ecarin clotting time, ACT: Activated clotting time, ROTEM: Rotational thromboelastometry, TEG: Thromboelastography, UFH: Unfractionated heparin. # 2–6 are plasma-based tests. # 1 and 7 are point of care whole blood tests, AC: Anticoagulant
| Factors associated with increased risk of thrombosis |
|---|
| Circuit/equipment • Low circuit flow • Insufficient volume • Pump speed • Blood flow velocity • Kinks or obstructions in the flow • Malpositioning • Large size of ECMO cannulas • Shear stress Patient factors • Prolonged duration of ECMO therapy • Advanced age • Sepsis • aPTT≤50 s • Elevated D-dimer levels • HIT And multitude of other factors that activate platelet and coagulation cascade may be contributing to this increased risk of cannula-associated DVT and VTE in general prolonged periods of time, advanced age, and those who had developed an infection during treatment |
ECMO: Extracorporeal membrane oxygenation, DVT: Deep vein thrombosis, VTE: Venous thromboembolism
Therapeutic monitoring of anticoagulants on ECMO
The best method to monitor the efficacy of both unfractionated heparin (UFH) and direct thrombin inhibitors (DTI) on ECMO is yet uncertain. This is because we are uncertain of the in vitro and in vivo responses of these drugs to the artificial ECMO endothelial surface. VET is a whole blood test and better than the aPTT type of tests which are plasma-based [Table 1]. The most commonly used tests for the assessment of the AC status of a patient on ECMO are ACT, activated prothrombin time (aPTT), anti-activated factor X assay (anti-Xa), and VET.[7] A brief description of each test is narrated below [Table 3].
| R time in the heparinase cuvette greater than 11 minutes | Give FFP |
| R time more than 2 minutes shorter in the heparinase cuvette | Give additional Protamine |
| If fibrinogen level was less than 100 mg.dL | Cryo precipitate was given |
| If Maximum Amplitude (MA) was less than 48 mm | Platelet |
ACT
ACT is a point-of-care test for assessing global hemostasis. The test utilizes whole blood and, therefore, acts as a functional test for the effects of coagulation factors and platelets. Its results can be confounded by the presence of anemia, thrombocytopenia, hypofibrinogenemia, GP IIb/IIIa antagonists, hemodilution, and hypothermia. It is the most commonly used point-of-care test to monitor the state of anticoagulation in ECMO patients.[8]
aPTT
aPTT is a plasma-based test for assessing both the intrinsic and common coagulation pathways. The results are institution or laboratory-specific and, hence, the results are to be analyzed with caution.
In most laboratories, the normal range is approximately 25–35 s. One of its advantage is that its results are not affected by hematocrit or platelet count. Its value has been found to correlate better with UHF concentrations during ECMO support than ACT levels. aPTT is a sensitive test for monitoring UFH concentration at the lower range (0.1 and 1 U/mL) while ACT is more useful at the higher range (1–5 U/mL).[9] Its normal range on ECMO is 45–60 s. Rajsic et al. did not find strong evidence supporting the aPTT-guided monitoring during ECMO. Based on the weight of the available evidence, further randomized trials are crucial to clarify the best monitoring strategy.[10]
Anti-Xa assay monitoring
Anti-Xa assay provides a functional assessment of the ability of UFH to catalyze the inhibition of Xa by antithrombin III (AT). The results can be falsely affected by hyperbilirubinemia, hyperlipidemia, and high plasma-free hemoglobin. Chromogenically measured anti-factor Xa activity in IU is equated to UFH levels based on protamine sulfate titration. Therefore, it is only a measure of the “heparin effect” and not other coagulation parameters.[11]
Role of VET in monitoring heparin anticoagulate: AIIMS experience (2018–2023)
VET such as Sonoclot, thromboelastography, and rotational thromboelastometry (ROTEM) measures the ability of whole blood to clot. They take into account, the property of a whole blood sample to clot under low shear stress. This allows the platelet function to be measured along with ongoing fibrinolysis. They can help to determine the need for appropriate blood components using a single test on ECMO and, thus, do away with packed red blood cells (RBCs) transfusion, when the hematocrit remains maintained on ECMO.[12,13]
In a study done at our institute (2016–2023) on 68 patients, the authors have discovered in a TOF patient on VA ECMO a signature curve on Sonoclot, showing poor platelet function, and needing as many as 10 numbers of single donor platelet (SDP) over 24 hours on VA ECMO. Reexploration was thus prevented for same, when bleeding subsided with SDP transfusion while ECMO continued. Patient improved and on 14 patients we observed platelet function on Sonoclot (Pfson) to be a good prognostic parameter for VA ECMO bleeding. The Sonoclot parameters Pfson alongwith, FIBTEM on ROTEM was also found to be an excellent VET to be done at the bedside on cyanotic patients. The Sonoclot Signatures done in cyanotic congenital surgery patients on VA ECMO at our center are shown in Figure 5a-e, showcasing the normal causes of bleeding/ thrombosis on VA ECMO, such as platelet dysfunction, hyperfibrinolysis, hyperfibrinogenemia, or heparin influence.
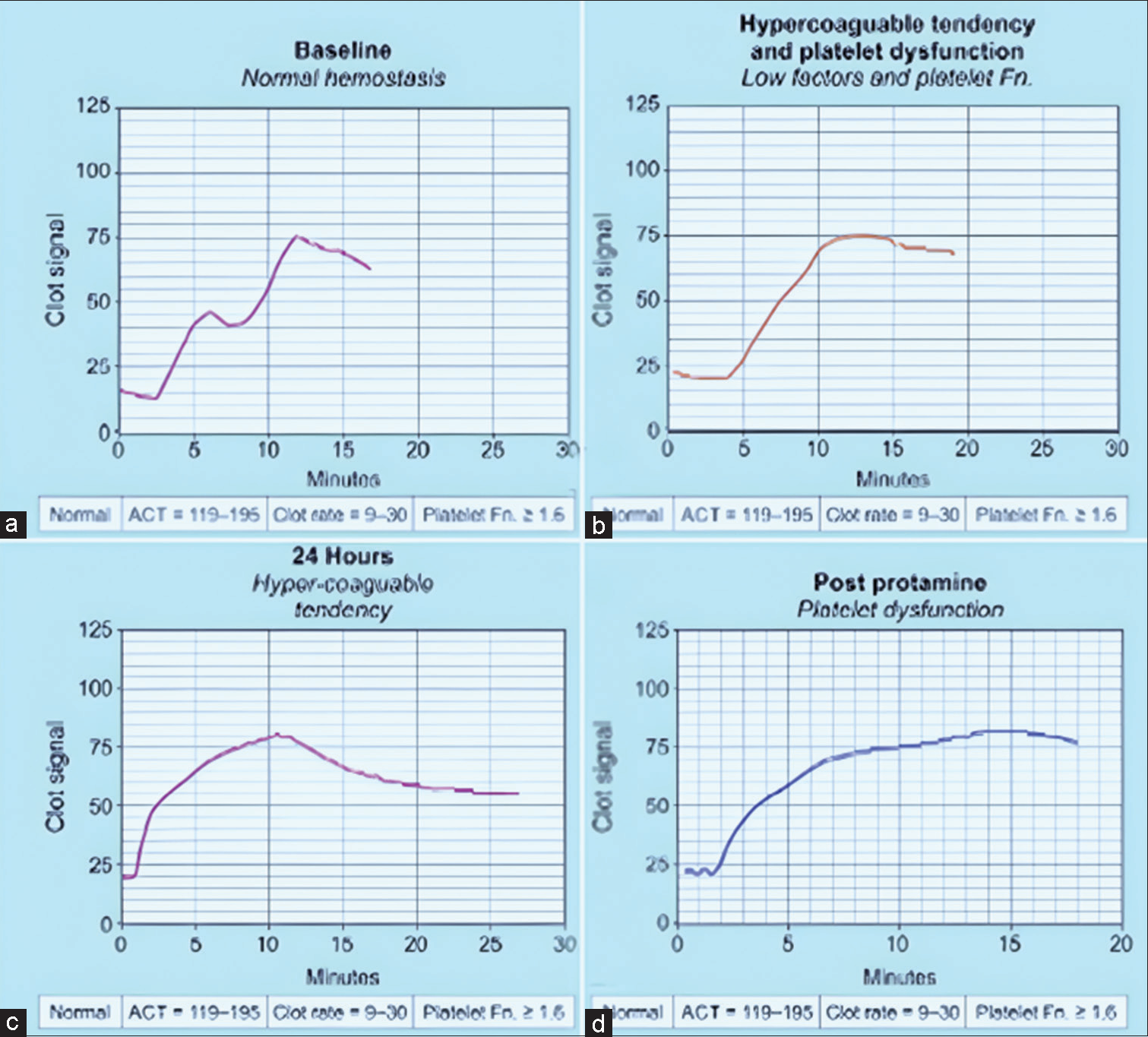
- (a-d) Sonolcot curve with platelet dysfunction in a Tetralogy of Fallot patient on venoarterial extracorporeal membrane oxygenation (ECMO) showing four different scenarios. (a) Normal hemostasis, (b) Low factors and poor platelet function, (c) Hypercoagulable tendency, signature curve on Sonoclot (d) Post-protamine for poor platelet function in a post-TOF patient on ECMO.
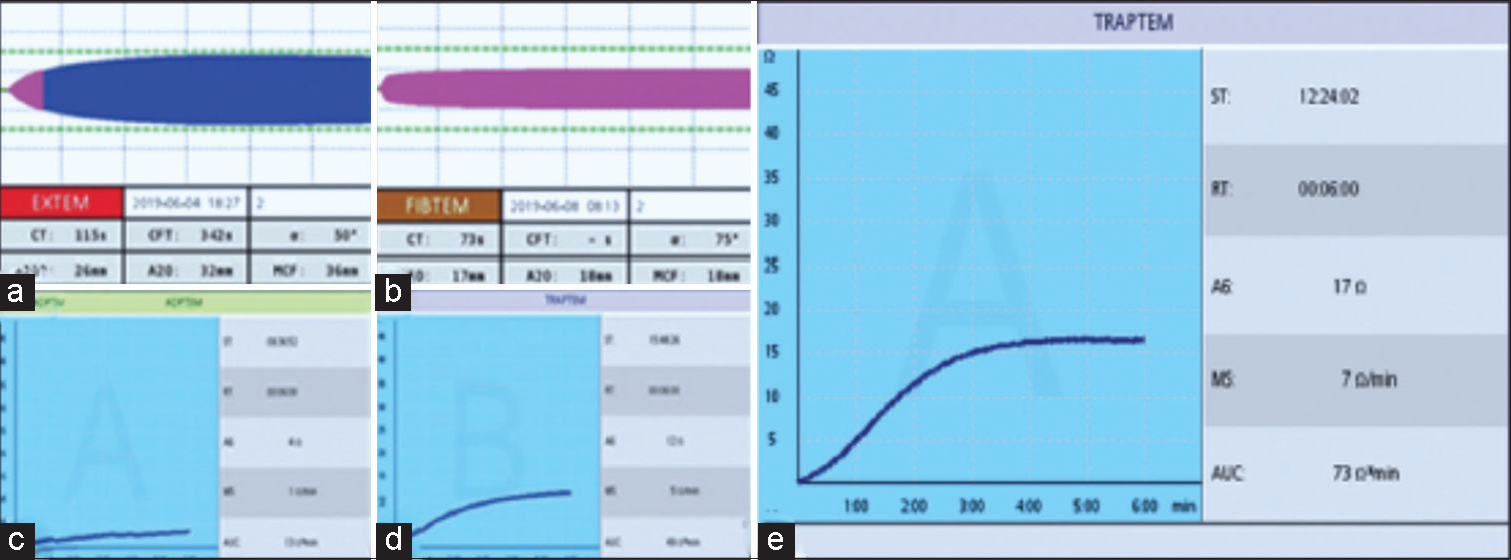
- (a-e) The use of rotational thromboelastometry (ROTEM) based viscoelastic test (VET) and platelet function tests (PFTs) to guide and monitor the correct component therapy. Figure a and b are cases of heparin infusion needing only protamine, showcasing heparin influence which necessity stopping heparin and giving protamine. Anticoagulation in extracorporeal membrane oxygenation (ECMO), done on venoarterial ECMO patients, shows the importance of ROTEM-based VET such as CT EXTEM and ADPTEM PFTs to detect the bleeding causes and factor deficiency. (e) shows good area under the curve in 10 minutes using TRAPTEM PFT reagent. This implies good platelet function, so no platelet transfusion is needed.
Platelet Function Tests in CABG on ECMO
In adult coronary artery bypass grafting patients on ECMO, we performed a platelet function test (PFT), as shown in [Figure 4a-d]. We observed, postoperatively in the intensive care unit (ICU) when on VA ECMO, a low-dose protamine with platelet concentrates to be the optimal therapy to give to these bleeding ECMO patient, rather than transfuse packed RBC whole blood, which may be harmful and cause adverse lung, injury (TRALI).
The AIIMS experience in a nutshell
At AIIMS, since its inception in 2002, complications of bleeding on VA ECMO, have reduced. Latter is chiefly due to timely insertion of ECMO (the use of integrated ECMO), the reduction in the heparin dosage with prolonged duration of ECMO, use of bivalirudin in ECMO patients with Heparin-induced thrombocytopenia (HITT), and routine use of Sonoclot / or ROTEM in a bleeding ECMO patient. Furthermore, judicious use of blood and blood products in a restricted manner helps prevent transfusion problems. ECMO has to be conducted in a systematic and protocol-based way to ensure safety and troubleshooting timely. For this, an institute must devise their own algorithms to follow the ROTEM parameters and interpret them stepwise giving the right component therapy when needed [Figure 5] [Tables 3 and 4].[14-20]
| ROTEM tests: Parameters that diagnose faster and more accurate bleeding | Major observations on ROTEM during ECMO – In AIIMS patients adapted from our own references |
|---|---|
|
|
CT: Clotting time, ML: Maximum lysis, ROTEM: Rotational thromboelastometry, VA ECMO: Venoarterial extracorporeal membrane oxygenation, ECMO: Extracorporeal membrane oxygenation, VET: Viscoelastic test, MCF: Maximum clot firmness
ALGORITHMS PAVED THE WAY TO GUIDE RIGHT THERAPY [FIGURE 5]
A total of 68 patients on VA ECMO were detected by TORCH test for thrombus. Thrombosis in 14 of 68 patients with the VET use and in one patient on bivalirudin infusion. aPTT and ACT alone did not detect any thrombosis while on VA ECMO, up to 48 hours of heparin/bivalirudin initiation. Algorithms to showcase, which blood component therapy to use, and at what VET test observation, helped us to better our results. Some of our observations are shown in Table 3 (on TEG) and Table 4 (on ROTEM - platelet aggregometry) [Tables 3 and 4].
THROMBOSIS IN THE ECMO CIRCUIT IS A COMMON OCCURRENCE ON ECMO AND IS A DISASTER TOOL: CLOSE VIGILANCE FOR CLOTS IS ESSENTIAL BY ALL IN THE ICU
The reported incidence of thrombosis in an ECMO circuit varies from 10% to 81%.[21] Once clotting is initiated on the artificial EC surface, it continues to propagate over time. Blood flow resistance declines and the oxygenator and pump begin to fail. Visual inspection alone with the torch test tends to underestimate the incidence of circuit thrombosis. Examination of the ECMO circuit’s post-removal has revealed thrombi in 34% oxygenators and 56% tubings and pumps. Visual inspection along with monitoring for changes in the patient’s coagulation profile, requires certain hematological parameters, and changes in pressure and blood gases across the oxygenator can help predict about 50% of the cases with acute mechanical ECMO failure including thrombosis of the oxygenator or blood pump, thus converting an emergency into an elective situation.[22] A local institutional troubleshooting algorithm [Figure 6] should be devised to aid in the prevention of excessive bleeding or thrombosis [Figure 7].[23-25]
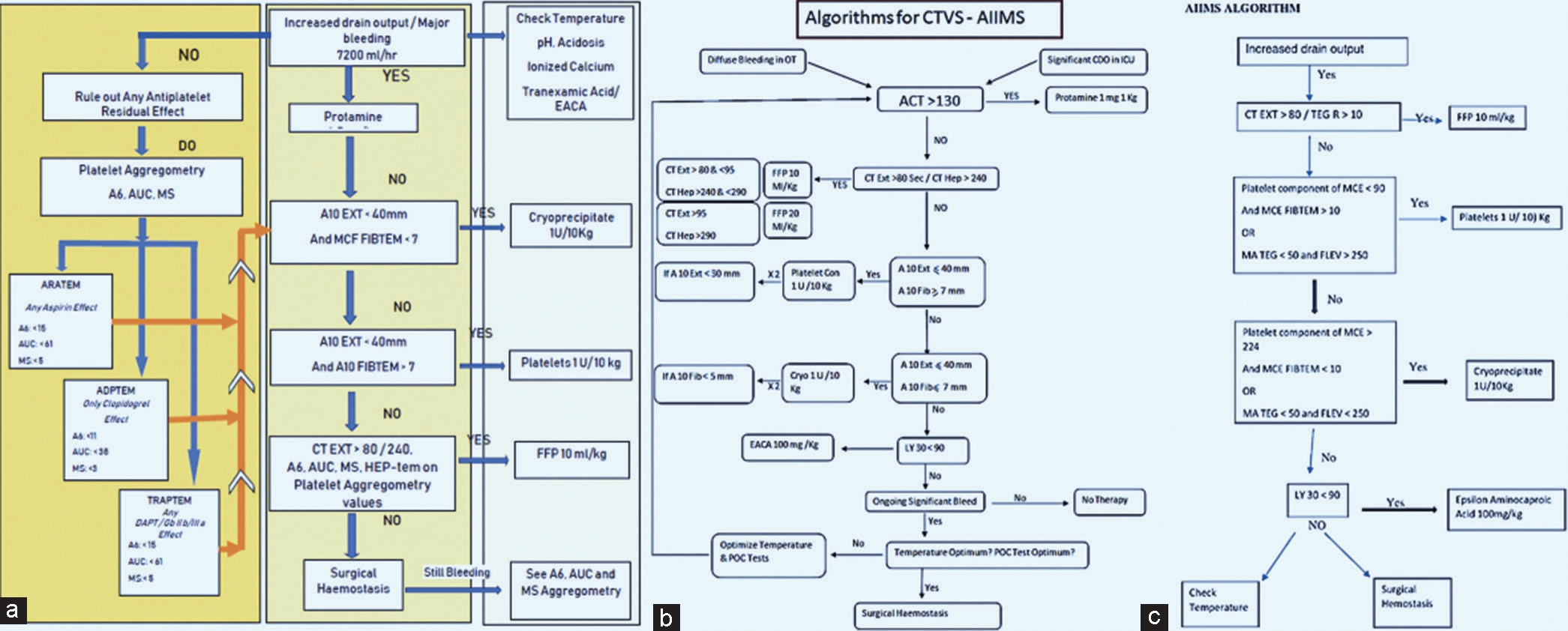
- (a) Algorithms for detecting and managing coagulation and bleeding problem on venoarterial extracorporeal membrane oxygenation, followed at AIIMS in adult coronary artery bypass grafting patients and (b and c) pediatrics patients.

- Bivalirudin thrombosis in the veno arterial extracorporeal membrane oxygenation (ECMO) Circuit seen with bivalirudin anticoagulation with cardiohelp oxygenator patient, a known case of HITT survived by stopping all anticoagulation and changing the ECMO circuit.
BIOLINE AND HEPARIN-BONDED CIRCUITS ARE NEED OF THE HOUR
At present, available commercial ECMO circuits are mostly some combination of the first two categories, for example, Bioline by Maquet, which has human albumin coating along with heparin bonded into the circuit.[26,27] With the use of bioline and heparin bonded circuits, there is a reduction in inflammatory response, fibrinolysis, and thrombocytopenia recued bleeding, thus a reduction in blood product usage.[28,29] Heparin-bonded surfaces are currently most popular commercially.[30] Evidence for clinical efficacy or impact on patient outcomes is frequently conflicting or comes from poor quality evidence as blinded, randomized trials are nearly impossible to conduct.[31]
WHAT IS NEEDED TO PREVENT THROMBOSIS?
VA ECMO bleeding may need surface modification
Altering the physicochemical properties (hydrophobicity or hydrophilicity) of the membrane surface, blunting the hemostatic response: For example, the extracorporeal circuit surface is coated with phosphorylcholine, albumin, or poly-2-methoxyethyl acrylate
AC graft onto the extracorporeal surface: Here, the extracorporeal surface is coated with heparin, nitric oxide, or DTI
Endothelialization of the blood-contacting surface.
ROTEM and bivalirudin: The confounding challenges ahead
In three of our VA ECMO patients treated with bivalirudin anticoagulation [Figures 8-10], we saw prolonged CT in INTEM and HEPTEM as markers of platelet dysfunction and thrombosis on VA ECMO. Some were quoted recently in the literature by Parastatidou et al.[32] Prolongation of CT on INTEM and HEPTEM has shown a moderate to strong correlation with traditional laboratory tests such as aPTT and HPTT (aPTT with Hepzyme).[18] ROTEM can be a way forward for monitoring bivalirudin therapy.[33]
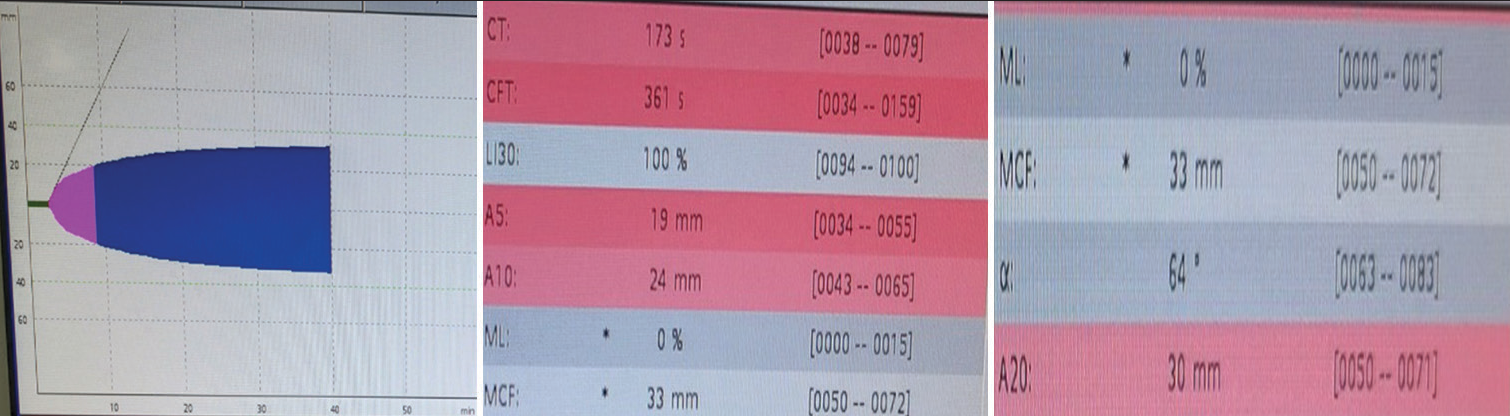
- ROTEM in bivalirudin thrombosis: EXTEM parameters showed a prolonged clotting time of 173 s, clot formation time of 361 s and decreased A5, A10, and A20 parameters.
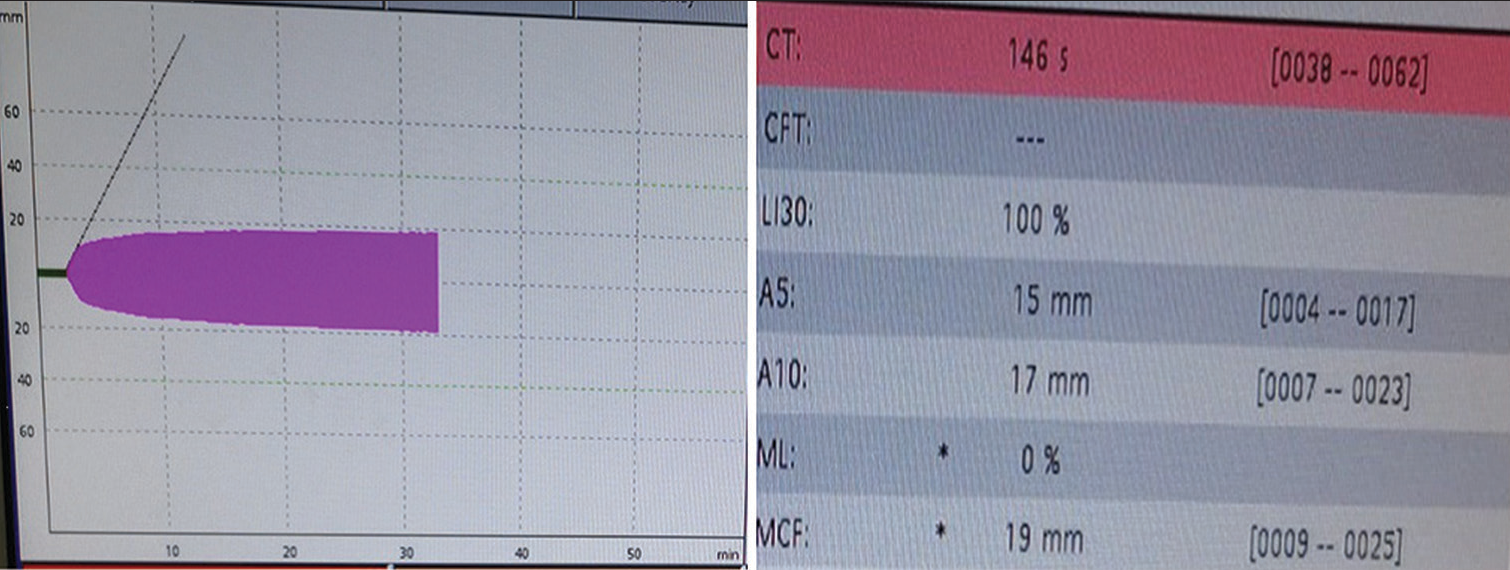
- ROTEM in bivalirudin thrombosis: The FIBTEM analysis showed a prolonged clotting time of 146 s with a normal A5 and A10 of 15 mm and 17 mm, respectively, and an MCF value of 19 mm implying a good hemostatic reserve and pointing toward a thrombocytopenic picture.
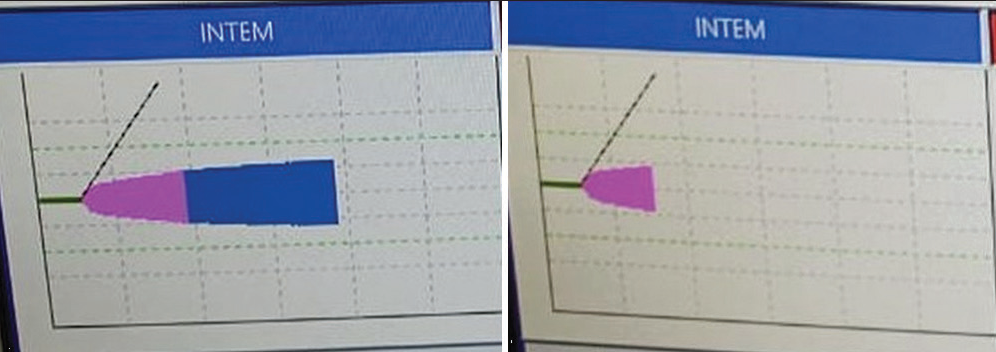
- ROTEM in bivalirudin thrombosis: In INTEM and HEPTEM clotting time prolonged both pointing toward platelet dysfunction as shown in prolonged CT.
CONCLUSION
While conducting VA ECMO on a cardiac surgical patient, a tailored approach rather than a generalized approach is preferred. Even though heparin is the most widely used anticoagulant, other heparin adjuncts like bivalirudin should be used in an ECMO Center.
Monitoring during ECLS is of paramount importance in diagnosing coagulopathy which helps in judicious use of blood and blood products and also early diagnosis of life-threatening thrombotic complications. No universally accepted protocols for monitoring anticoagulation on ECMO exist, but we all universally follow the ELSO guidelines updated in 2021;[34] usually, in which the authors reiterate the use of a combination of tests to detect the coagulopathy.[35-37]
Extensive research on modifications of extracorporeal surfaces is ongoing, few are applicable outside the laboratory. Each ECMO unit should formulate its algorithm based on experience, cost, complication rates and outcomes, and patient factors. Such algorithms should include the risk of bleeding and thrombosis, which requires VET monitoring and interpretation in expert hands. Timely ECMO insertion and anticoagulation use is the key to preventing both bleeding and thrombosis on ECMO. VET should be used timely and routinely to prevent the occurrence of both on VA ECMO. VET and PFTs are not done routinely in most ECMO centers and it is time to change this.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Poonam Malhotra Kapoor is the member of the Editorial Board.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Poisoning: Kinetics to Therapeutics In: Critical Care Nephrology (3rd ed). Netherlands: Elsevier Inc; 2019. p. :600-29.
- [CrossRef] [Google Scholar]
- Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017;63:60-7.
- [CrossRef] [PubMed] [Google Scholar]
- Current Understanding of how Extracorporeal Membrane Oxygenators Activate Haemostasis and other Blood Components. Front Med (Lausanne). 2018;5:352.
- [CrossRef] [PubMed] [Google Scholar]
- Blood Coagulation and its Regulation by Anticoagulant Pathways: Genetic Pathogenesis of Bleeding and Thrombotic Diseases. J Intern Med. 2005;257:209-23.
- [CrossRef] [PubMed] [Google Scholar]
- Anticoagulation during ECMO: Will the Tight Rope be Tighter in 2018? J Cardiac Crit Care TSS. 2017;1:55-6.
- [CrossRef] [Google Scholar]
- ELSO Guidelines for Adult and Pediatric Extracorporeal Membrane Oxygenation Circuits. ASAIO J. 2022;68:133-52.
- [CrossRef] [PubMed] [Google Scholar]
- Activated Clotting Time (ACT) Methods Mol Biol. 2013;992:155-67.
- [CrossRef] [PubMed] [Google Scholar]
- Anticoagulation in ECMO Patients: An Overview. Indian J Thorac Cardiovasc Surg. 2021;37:241-7.
- [CrossRef] [PubMed] [Google Scholar]
- Association of aPTT-Guided Anticoagulation Monitoring with Thromboembolic Events in Patients Receiving V-A ECMO Support: A Systematic Review and Meta-analysis. J Clin Med. 2023;12:3224.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-factor Xa-based Anticoagulation during Extracorporeal Membrane Oxygenation: Potential Problems and Possible Solutions. Semin Thromb Hemost. 2020;46:419-27.
- [CrossRef] [PubMed] [Google Scholar]
- Viscoelastic Testing in the Management of Adult Patients on Mechanical Circulatory Support Devices with Focus on Extracorporeal Membrane Oxygenation. Semin Thromb Hemost. 2022;48:814-27.
- [CrossRef] [PubMed] [Google Scholar]
- Coagulopathies in Cyanotic Cardiac Patients: An Analysis with Three Point-of-Care Testing Devices (Thromboelastography, Rotational Thromboelastometry, and Sonoclot Analyzer) Ann Card Anaesth. 2017;20:212-8.
- [CrossRef] [PubMed] [Google Scholar]
- Simulation in Thromboelastography: Platelet Mapping Assay (Part-I) Ann Card Anaesth. 2016;19:530-2.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet Aggregometry Interpretation using ROTEM-PART-II. Ann Card Anaesth. 2016;19:584-6.
- [CrossRef] [PubMed] [Google Scholar]
- A Prospective Randomized Clinical Trial of Efficacy of Algorithm-Based Point of Care Guided Hemostatic Therapy in Cyanotic Congenital Heart Disease Surgical Patients. J Card Crit Care TSS. 2019;3:8-16.
- [CrossRef] [Google Scholar]
- Use of Integrated Extracorporeal Membrane Oxygenator in Anomalous Left Coronary Artery to Pulmonary Artery: Better Survival Benefit. Ann Card Anaesth. 2011;14:240-2.
- [CrossRef] [PubMed] [Google Scholar]
- ROTEM and Bivalirudin: The Confounding Challenges Ahead. J Card Crit Care TSS. 2023;7:71-4.
- [CrossRef] [Google Scholar]
- Evaluation of Coagulopathy on Veno-arterial ECMO (VA) Extracorporeal Membrane Oxygenation using Platelet Aggregometry and Standard Tests: A Narrative Review. Egypt J Crit Care Med. 2018;6:73-8.
- [CrossRef] [Google Scholar]
- Role of Extracorporeal Membrane Oxygenation for Acute Decompensated Heart Failure. In: Manual of ECMO in the ICU (1st ed). New Delhi: Jaypee Publishers; 2014. p. :188-92.
- [CrossRef] [Google Scholar]
- Modification Strategies to Improve the Membrane Hemocompatibility in Extracorporeal Membrane Oxygenator (ECMO) Adv Compos Hybrid Mater. 2021;4:847-64.
- [CrossRef] [PubMed] [Google Scholar]
- Runtime and aPTT Predict Venous Thrombosis and Thromboembolism in Patients on Extracorporeal Membrane Oxygenation: A Retrospective Analysis. Ann Intensive Care. 2016;6:66.
- [CrossRef] [PubMed] [Google Scholar]
- Technical-Induced Hemolysis in Patients with Respiratory Failure Supported with Veno-venous ECMO-Prevalence and Risk Factors. PLoS One. 2015;10:e0143527.
- [CrossRef] [PubMed] [Google Scholar]
- Reexploration can be Deterred by Point-of-Care Testing in Cardiac Surgery Patient. J Card Crit Care TSS. 2017;1:48-50.
- [CrossRef] [Google Scholar]
- Extracorporeal Membrane Oxygenation: Anticoagulation. In: Manual of ECMO in the ICU (1st ed). New Delhi: Jaypee Publishers; 2014. p. :109-11.
- [CrossRef] [Google Scholar]
- Do we Need Heparin Coating for Extracorporeal Membrane Oxygenation? New Concepts and Controversial Positions about Coating Surfaces of Extracorporeal Circuits. Artif Organs. 2015;39:176-9.
- [CrossRef] [PubMed] [Google Scholar]
- The Effects of Carmeda Bioactive Surface on Human Blood Components during Simulated Extracorporeal Circulation. J Thorac Cardiovasc Surg. 1996;111:1073-84.
- [CrossRef] [PubMed] [Google Scholar]
- Significantly Reduced Adsorption and Activation of Blood Components in a Membrane Oxygenator System Coated with Crosslinkable Zwitterionic Copolymer. Acta Biomater. 2016;40:153-61.
- [CrossRef] [PubMed] [Google Scholar]
- A Prospective, Double-blind Study on the Efficacy of the Bioline Surface-heparinized Extracorporeal Perfusion Circuit. Ann Thorac Surg. 2003;76:129-35.
- [CrossRef] [PubMed] [Google Scholar]
- Biocompatibility of Heparin-coated Extracorporeal Bypass Circuits: New Heparin Bonded Bioline System. Artif Organs. 2000;24:618-23.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-thrombogenic Surface Coatings for Extracorporeal Membrane Oxygenation: A Narrative Review. ACS Biomater Sci Eng. 2021;7:4402-19.
- [CrossRef] [PubMed] [Google Scholar]
- The Role of ROTEM Variables based on Clot Elasticity and Platelet Component in Predicting Bleeding Risk in Thrombocytopenic Critically Ill Neonates. Eur J Haematol. 2021;106:175-83.
- [CrossRef] [PubMed] [Google Scholar]
- Rotational Thromboelastometry (ROTEM(®)) in Relation to Inflammatory Biomarkers and Clinical Outcome in COVID-19 Patients. J Clin Med. 2023;12:3919.
- [CrossRef] [PubMed] [Google Scholar]
- Patient Blood Management in Cardiac Surgery and ECMO: The Indian Scenario in 2021. J Card Crit Care. 2021;5:181-3.
- [CrossRef] [Google Scholar]
- 2021 ELSO Adult and Pediatric Anticoagulation Guidelines. ASAIO J. 2022;68:303-10.
- [CrossRef] [PubMed] [Google Scholar]
- Role of Platelet Function Test in Predicting Postoperative Bleeding Risk after Coronary Artery Bypass Grafting: A Prospective Observational Study. J Card Crit Care TSS. 2021;5:88-96.
- [CrossRef] [Google Scholar]
- Simulation in Coagulation Testing using Rotational Thromboelastometry: A Fast Emerging, Reliable Point of Care Technique. Ann Card Anaesth. 2016;19:516-20.
- [CrossRef] [PubMed] [Google Scholar]






