Translate this page into:
Understanding Heart Transplantation—Gift of Life
Poonam Malhotra Kapoor, MD, DNB, MNAMS, FIACTA (Hony), FTEE (Hony), FISCU (Hony) Department of Cardiac Anaesthesia, All India Institute of Medical Sciences (AIIMS), New Delhi–110029 India docpoonamaiims@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Heart transplantation (HTx) provides a longer and better quality of life to patients with end-stage heart failure when all other options of treatment have been exhausted. There are nearly 500 heart donors yearly in India. Approximately 60 cases of HTx are performed every year; 70% survival rate is for nearly 1 year whereas 30% survival rate is for 10 years. Scarcity of donors is further complicated by the use of single organ, heart injury with common brain-death injuries, difficulty with ex vivo preservation, heart disease among donors, and complexity of the operation.
Keywords
cardiac anesthesia
end-stage heart failure
heart transplant
Introduction
Heart transplantation (HTx) provides a longer and better quality of life to patients with end-stage heart failure when all other options of treatment have been exhausted.1 There are nearly 500 heart donors yearly in India. Approximately 60 cases of HTx are performed every year; 70% survival rate is for nearly 1 year whereas 30% survival rate is for 10 years.
Scarcity of donors is further complicated by the use of single organ, heart injury with common brain-death injuries, difficulty with ex vivo preservation, heart disease among donors, and complexity of the operation.
Who Is Considered for Heart Transplant?
Patients with an intractable angina, severe symptoms of heart failure, or rhythm disturbances, without any alternative form of treatment with a poor prognosis as recommended by the ACC/AHA (American College of Cardiology/American Heart Association) guidelines and cardiac resynchronization therapy, are considered for HTx. Nonischemic cardiomyopathy (53%) is the most common cause for indication of HT. Other indications include valvular heart disease (3%), retransplantation (3%) and others (Table 1). Vo2 max is a dynamic objective variable used to evaluate potential transplant candidates and their long-term risk. Generally, a peak Vo2 > 14 mL/kg/min is insufficient indication for HTx as transplantation has not been shown to improve survival over conventional medical therapy and studies showed peak Vo2 < 10 had the greatest survival benefit.4, 5 Recently, it has been suggested that ventilatory efficiency (VE/Vco2) may be a more powerful prognostic factor than Vo2 max, as it is regardless of body mass index.
|
Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; HF, heart failure; ICD, implantable cardioverter defibrillator; LV, left ventricular; NYHA, New York Heart Association; PHT, pulmonary hypertension. (Circulation 2009; 119:1977–2016.) |
|
|
|
|
|
|
|
The conditions listed in Table 2 are generally considered contraindications for HTx.
|
Absolute contraindications3 |
|
|
|
|
|
|
Relative contraindications3 |
|
|
|
|
|
|
|
|
|
|
Evaluation of Cardiac Transplantation Recipient
Selection of potential cardiac recipients is not a simple process. A recipient must undergo a thorough evaluation in an attempt to identify any condition that determines the long-term survival after transplantation. Consider carefully scrutinizing the patient by physiologic age and condition. Prior to listing for transplantation, it is imperative for the patients to undergo mental health evaluation to identify any substance abuse, as noncompliance would lead to adverse post-transplant outcome. In the evaluation process, apart from routine biochemistry, certain tests are required to evaluate hemodynamics and, in particular, to evaluate for any reversible pulmonary hypertension component that precludes consideration from transplant. Patients should also undergo serologic test for cytomegalovirus, toxoplasmosis, Epstein-Barr virus, hepatitis B, and hepatitis C.
Cardiopulmonary testing is required to refine the risk stratification. Pretransplant immunologic evaluation includes ABO and Rh blood–type antibody screen for listing purposes as well as determination of the panel of reactive antibodies (PRAs) against HLA, which can make the transplant unsuccessful, as allosensitization to human leukocyte antigen (HLA) antibodies can pose a particular problem that may defer transplant eligibility. One should also gain information regarding potential sources of financial support.
Cardiac Donor
Donor selection is influenced by many factors, including age, presence of anti-HLA antibodies, cytomegalovirus (CMV) infection, comorbidities, ABO blood–type compatibility, size similarity between the donor and recipient, presence of intrinsic cardiac disease, and presence of transmissible infectious or malignant diseases. Metabolic alterations after the donor’s brain death mandate the donor to undergo electrocardiography, echocardiography, and sometimes coronary angiography, when indicated.
All potential donors should undergo a full echocardiographic examination, which is the most important tool for examination of donor heart function. In interpretation of the echocardiogram findings, however, particular attention should be paid to the presence of left ventricular hypertrophy (LVH), significant physiologic valvular dysfunction, and depressed ventricular function.
Additionally, once the consent is obtained for donation, donors are screened for ABO/Rh testing, human immunodeficiency virus (HIV), hepatitis, and CMV infection. The goal of donor care before organ harvesting is to ensure the optimal myocardial and end-organ perfusion. Donors with beating hearts are often volume depleted because of therapy directed at reducing cerebral edema. Close attention must be paid to maintain core temperature, because hypothermia adversely affects cardiac rhythm, coagulation, and oxygen delivery. To optimize the chance of success, there are a multitude of factors to determine the outcome, including ischemic time, recipient comorbidities, and condition at time of transplantation, size matching, and presence of PRAs.
Matching Donor and Recipient
Size matching between donor and recipient deserves special attention; however, size matching is based on more precise body mass index or height than weight alone. In patients with known pulmonary hypertension, extra caution must be needed not to undersize the donor heart size to the recipient by > 30% mismatch.
Because ischemic time during cardiac transplantation is crucial, an ischemic time of < 4 hours is optimal. The PRA is a rapid measurement of preformed reactive anti-HLA antibodies in the transplant recipient. In general, cross-matching is not required if PRA is < 10 to 20%. If PRA is > 20%, a T- and B-cell cross-match should be performed. Patients with an elevated PRA needs plasmapheresis, immunoglobulins, or immunosuppressive agents.6
Donor Cardiectomy—Organ Preservation
Before proceeding with cardiectomy, perfusion-sensitive organs (kidneys and liver) are removed after systemic heparinization. After dissection of the pericardial attachments and inspection of the heart, coronary arteries are palpated. Ascending aorta is cross-clamped for administration of cold cardioplegia. Topical cooling is achieved with ice-cold saline.
The superior vena cava (SVC) and inferior vena cava (IVC) are mobilized and ascending aorta dissected from the pulmonary artery. Finally, cardiectomy is completed by dividing four pulmonary veins and by dividing the pulmonary artery at its bifurcation and aorta (as high as possible) (Fig. 1). During organ retrieval and ischemic storage, hypothermia is the principal means of donor heart preservation aimed at minimizing graft dysfunction caused by ischemia-reperfusion injury.
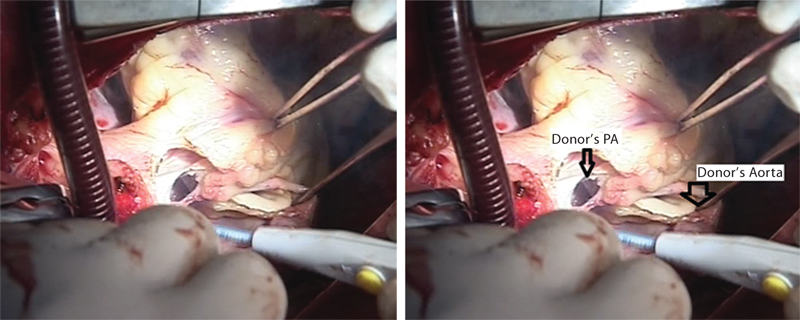
- Transected aorta. Donor’s pulmonary artery (PA) is being transected.
Hypothermia is maintained at 4 to 8°C as it markedly decelerates metabolism; the effectiveness depends on the cardioplegia solution and its temperature. Based on the potassium and sodium concentrations, preservation solutions can be broadly classified into intra- and extracellular solutions. The risk of hyperkalemia-induced pulmonary vasoconstriction with intracellular solutions favored the design of extracellular solutions. HTK (or Custodiol) as extracellular cardioplegic solution has subsequently been applied for cardiac preservation as well as for other organ preservation including the liver and kidney.
Single administration of cold Custodiol in the coronary vascular bed provides reliable protection of the heart for at least 2 hours.
Organ Care System
Organ care system (OCS) was the world’s first commercial, portable system designed to perfuse the donor hearts with warm, oxygenated, nutrient-rich blood, maintaining the organ in a beating, functioning state in transit. Advantages of OCS include less time-dependent ischemic injury to donor organs, ability to evaluate the functional status of donor organs outside the body, ability to resuscitate organs after removal from the donor, improved patient outcomes, increased use of available organs, expansion of the pool of potential donors, and reduced total cost of care (Fig. 2).

- Organ care system (OCS).
Preoperative Management of Transplant Recipient
A close communication between the transplant team and the organ retrieval team must be maintained to reduce prolonged donor heart ischemic times and recipient anesthesia time, because of adhesions and bleeding in recipients who have undergone prior cardiac surgery that will increase the surgical time to achieve cardiopulmonary bypass (CPB). Anesthetic induction should be timed so that CPB is commenced immediately upon donor heart arrival.
Diuretics may cause hypokalemia and hypomagnesemia, and hypovolemia increases risk of dysrhythmias. Preoperative inotropic support (amiodarone, milrinone, enoximone) should be continued throughout the pre-CPB period, but their toxic side effects increase mortality.7 The position, function, and duration of intra-aortic balloon counterpulsation and ventricular assist devices (VADs) should be noted. Current immunosuppressive protocol consists of combination of cyclosporine with prednisolone and mycophenolate mofetil (MMF). Immunosuppression begins preoperatively and is continued throughout life. Difficulties encountered during placement of an arterial catheter in patients with an axial flow left ventricular assist device (LVAD) mandate ultrasound devices as one cannot feel arterial pulse.8 Premedication should be carefully titrated or avoided as small dose of sedative may result in vasodilation and hemodynamic decompensation.
Apart from noninvasive monitoring (standard five-lead electrocardiogram [ECG], noninvasive blood pressure [BP] measurement, pulse oximetry, capnography, nasopharyngeal temperature, urinary output), placement of invasive monitoring should be commenced under all aseptic precautions, including central venous or pulmonary artery catheter, arterial line, transesophageal echocardiography (TEE), and cardiac output (CO) monitoring. CMV-negative blood products should be procured for patients lacking antibodies to this ubiquitous organism. Aseptic technique with broad-spectrum antibiotic prophylaxis should be administered.
Intraoperatively
Hemodynamic goals include maintaining heart rate (HR) and contractility, avoiding acute changes in preload and afterload, and preventing increases in pulmonary vascular resistance. Induction of anesthesia balances risk of aspiration of gastric contents with hemodynamic changes. High-dose narcotic with muscle relaxant and benzodiazepines are preferred. Most patients called in for transplantation have not fasted and should be considered to have a full stomach dictating the need for a rapid sequence induction with the preoperative use of agents to promote gastric emptying and drugs to raise gastric pH.
Induction should be performed in the presence of the surgeon, scrub nurse, and perfusionist in anticipation for cardiovascular collapse. Anticipate altered drug responses due to low CO and slow circulation time as well as decreased volume of distribution. Preinduction administration of inotropic agents or pressors optimizes circulation and minimizes transit time of subsequently administered anesthetics.
Considerations for repeat sternotomy in HTx recipients include potential for increased risk for inadvertent trauma to the great vessels, increased perioperative bleeding that often necessitates the need for femoral or axillary CPB cannulation, or injury to preexisting coronary grafts during redo-sternotomy, in addition to potential for a prolonged surgical dissection time. Therefore, it is mandatory to place external defibrillation pads and cross-matched, packed red blood cells (RBCs) available in the operating room beforehand.
Following individual cannulation of the vena cavae and aorta, CPB-instituted patients are cooled between 28 and 30°C and diseased heart is excised, leaving an atrial cuff containing the cavae, pulmonary veins, and remnants of the pulmonary artery and aorta.
Surgical Considerations
Orthotopic implantation is currently used in clinical practice. It involves complete explantation of the native heart. There are two types:
-
Biatrial anastomosis: It is most common because it allows a shorter ischemia time. Complications include atrial dysfunction due to size mismatch of atrial remnants and arrhythmia (sinus node dysfunction, bradyarrhythmias, and atrioventricular [AV] conduction disturbances), which necessitate permanent pacemaker implantation in 10 to 20% of patients (Fig. 3 Fig. 4 Fig. 5).
-
Bicaval anastomosis: It decreases incidence of arrhythmias and the risk for mitral or tricuspid regurgitation. However, narrowing of the SVC and IVC make biopsy surveillance difficult, and ischemic times can be prolonged (Fig. 6 Fig. 7 Fig. 8). Following median sternotomy, aortic cannulation is accomplished with straight cannula high in the ascending aorta. The ascending aorta is cross-clamped just proximal to the aortic cannula and excision of the diseased heart proceeds.
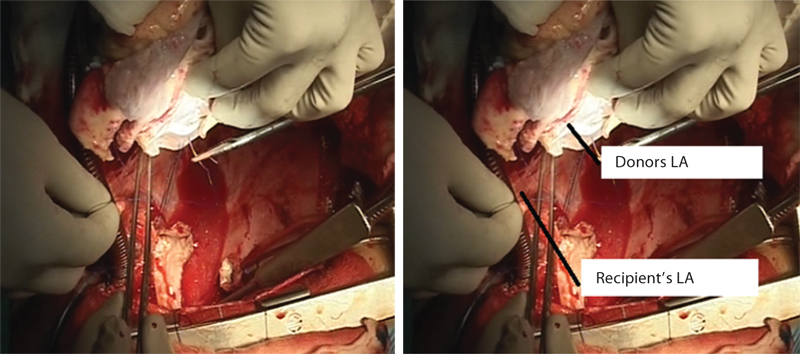
- Donor’s left atrium (LA) is being sewn into the recipient’s LA.
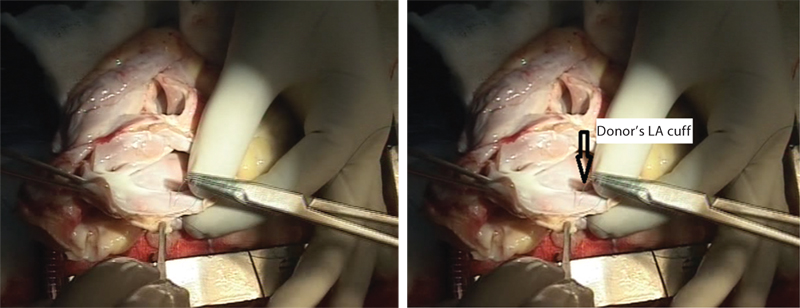
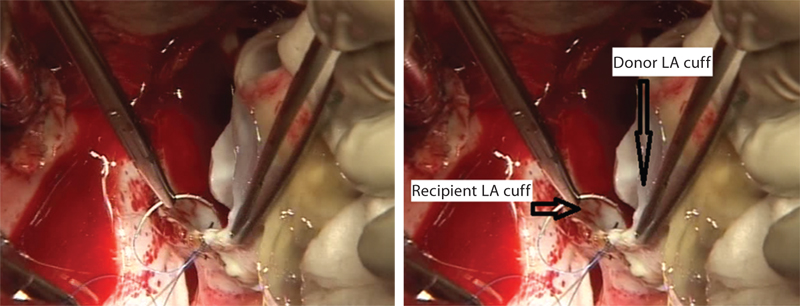
- Sutures are being taken on donor’s left atrial cuff for anatomosis into recipient’s left atrium (LA).
- Donor’s left atrium (LA) is being sewn into the recipient’s LA.
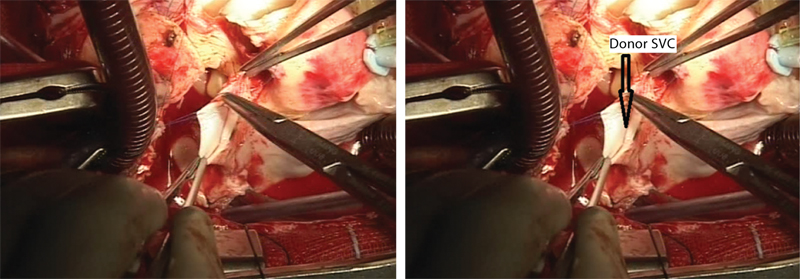
- Donor’s superior vena cava (SVC) is being sewn into the recipient’s SVC.
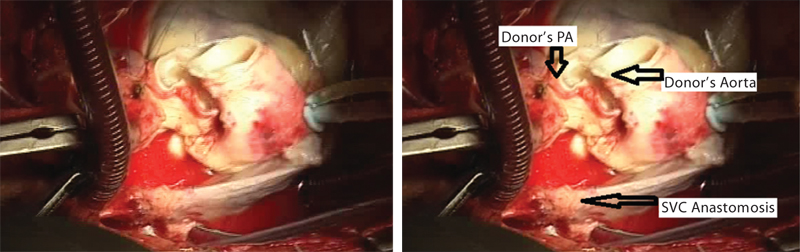
- Final anastomosis of superior vena cava (SVC). PA, pulmonary artery.
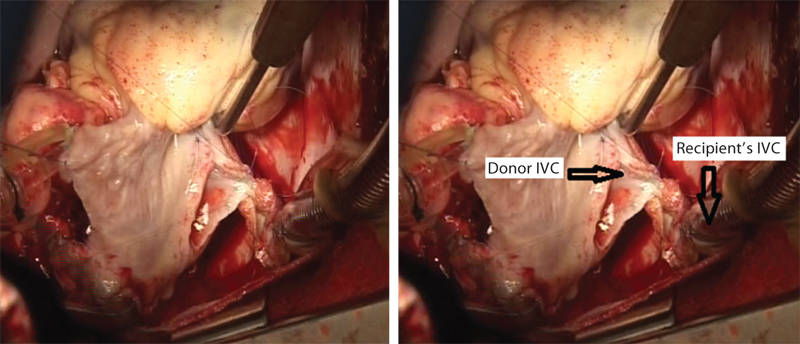
- Donor’s inferior vena cava (IVC) is being sewn into the recipient’s IVC.
Aorta and pulmonary artery are transected just above the respective semilunar valves. In certain situations, recipient instability during anesthetic induction or mediastinal dissection may dictate femoral cannulation for the institution of CPB. The order of anastomoses is the left and right atrial anastomoses, the end-to-end pulmonary anastomoses, and finally aortic anastomosis (Fig. 1, 3 4 5 6 7 8 9 10). Once the aortic anastomosis has been accomplished, an air-vent site is created in the most anterior portion of the ascending aorta; the patient is placed in Trendelenburg’s position. The air-vent site in the ascending aorta is left open while ventilation is initiated, and maneuvers are performed to remove air from the left heart. When the patient has returned to normothermia and at least 30 minutes of reperfusion has occurred, isoproterenol infusion is initiated.
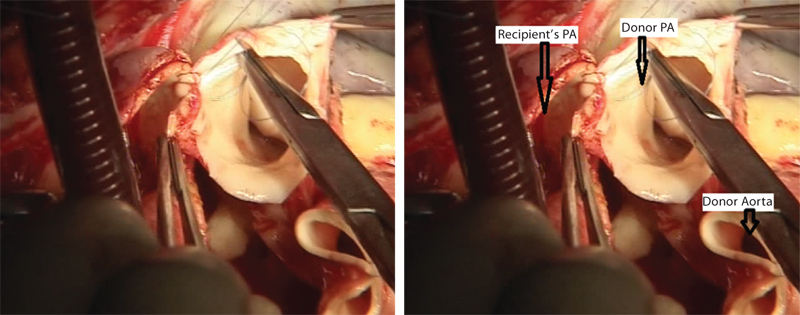
- Donor’s pulmonary artery (PA) is being sewn into the recipient’s PA.
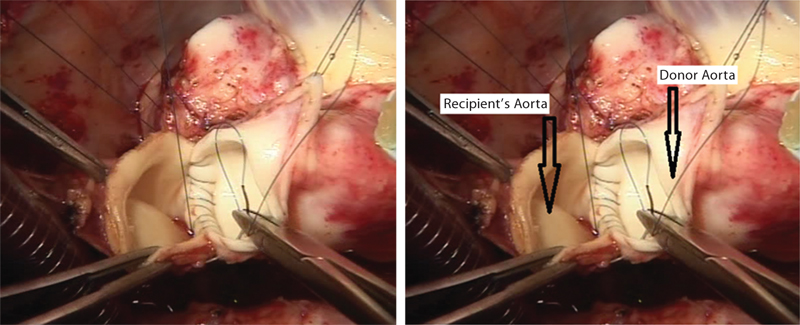
- Donor’s aorta is being sewn into the recipient’s aorta.
Glucocorticoid (methylprednisolone 500 mg) is administered as the last anastomosis is being completed prior to the release of the aortic cross clamp to attenuate any hyperacute immune reaction.
After termination of CPB, TEE may be of particular value if cardiac chambers are adequately de-aired and can diagnose atrial torsion, right ventricular (RV) outflow obstruction, and right or left ventricular systolic function. During reperfusion, an infusion of an inotrope is begun for both inotropy and chronotropy. Donor heart should be paced if there is persistent bradycardia despite inotrope support. There is a raised possibility of intra-aortic balloon pump (IABP), extracorporeal membrane oxygenation (ECMO), or LVAD, as RV dysfunction from elevated PVR is the most common cause of perioperative heart failure. Use of pulmonary vasodilators, such as milrinone, nitric oxide, and sodium nitroprusside, is advised. Arrhythmias such as slow junctional or AV nodal and V fib are common.
Postoperatively
Low CO after transplant may occur due to hypovolemia, inadequate adrenergic stimulation, myocardial injury during harvesting, acute rejection, tamponade, and sepsis. Risk factors for low CO include prolonged donor ischemia time, inadequate myocardial protection, and intracoronary air. Bleeding can be significant if the patient has been anticoagulated for circulatory assist device particularly for a prolonged period of time. Other risk factors include preoperative anticoagulation, preoperative liver dysfunction, long CPB time, and hypothermia.
To avoid leukocyte-mediated adverse reactions, leukodepleted blood transfusion is preferred. Post-transplant arrhythmias are common in early postoperative period, particularly in orthotopic HTx recipient. Dysrhythmias such as bradycardia, AV node dysfunction are especially frequent and may result in significant morbidity or mortality. Pacing and chronotropic agents may be required for several weeks. Early postoperative bradyarrhythmias due to sinus node dysfunction can be reduced by the bicaval technique.
Renal failure is a common complication following HTx, associated with short-term survival. Risk factors include preoperative impaired renal function and perioperative hypotension CPB. This risk is again compounded by major immunosuppressive agents.9
Early Complications
Primary Graft Failure
Primary graft dysfunction (PGD) is a common occurrence after transplant. PGD is a life-threatening complication occurring within the first 24 hours of completion of surgery of HTx that presents as LV dysfunction, RV dysfunction,10 or biventricular dysfunction without any identifiable secondary cause. Donor, recipient, and surgical procedural factors are contributing risk factors for PGD.
PGD may manifest from mild to severe depending on the extent of myocardial dysfunction and hemodynamic instability, as well as the extent of myocardial support required to maintain hemodynamics and perfusion to vital organs.
The pathophysiology is not well defined, but it is believed that acute catecholamine toxicity and the release of multiple proinflammatory mediators in the donor, followed by ischemia-reperfusion injury during retrieval and transport, contribute to development of PGD. A potential approach in management includes minimization of risk factors, gradual increase of inotropes, and use of mechanical circulatory support, as needed.11
Right Ventricle Dysfunction
RV dysfunction accounts for 20% early posttransplant death in patients with preexisting pulmonary hypertension. Right ventricle is subjected to similar ischemic or reperfusion injury risks as the left ventricle; hence, strategies to prevent RV dysfunction include avoid hypoxia, hypercarbia, acidosis, hypothermia, RV dilation, and the failure of coaptation of the TV leaflets, leading to severe TR. The rationale of treatment is optimization of RV preload, avoiding overdistention and underfilling, administration of inotropic and chronotropic support such as milrinone and dobutamine, maintaining the coronary perfusion with help of vasopressors, and reducing the PVR using nitrates, prostaglandin, and nitric oxide (NO).
Infections
Infection is a leading cause of late mortality (130 days posttransplantation surgery). Bacterial pneumonia is very common in the early postoperative period, and opportunistic viral and fungal infections occur after the first several weeks.12 CMV is one of the most important pathogens affecting the long-term outcome of transplant recipients.13 Two approaches can be differentiated regarding the prevention of CMV infection: preemptive therapy and prophylaxis. Currently, prophylaxis and preemptive therapy with ganciclovir are indicated.
Rejection
According to ISHLT (International Registry for Heart and Lung Transplantation) registry, 30% rejection occurs during first year. Three types of observed rejection include hyperacute, acute, and chronic. Hyperacute rejection occurs after reperfusion, caused by preformed antibodies against the donor in the recipient.14 It occurs within minutes to hour. The best method for avoiding hyperacute rejection is PRA screening. Acute cellular rejection occurs in almost 50% of cardiac transplant recipients, mediated by anti-HLA antibodies.
Half of all episodes occur within the first 2 to 3 months.15 It is rarely observed beyond 12 months unless immunosuppressant has been decreased. Rejection may manifest as nonspecific constitutional symptoms, features of myocardial inflammation. Rejection of the cardiac allograft is usually clinically silent unless it is accompanied by significant hemodynamic compromise (i.e., congestive heart failure) in a more evident form.
Chronic rejection in the form of cardiac allograft vasculopathy is one of the major factors that affect long-term graft and patient survival after HTx. An endomyocardial biopsy is the gold standard for early diagnosis of rejection.16
Late Complications
Cardiac Allograft Vasculopathy
Cardiac allograft vasculopathy is characterized by diffuse, concentric intimal hyperplasia of coronary arteries associated with acute antibody-mediated rejection and may be asymptomatic, or presents as silent myocardial infarction (MI), sudden death, and progressive heart failure. It is due to an immune cell-mediated activation of vascular endothelial cells to upregulate the production of smooth muscle cell growth factors.17
Risk factors that should be taken into account include donor’s age, recipient’s age, systemic arterial hypertension, and earlier transplant. These patients are evaluated by angiography, but this modality may underestimate the degree of diffuse intimal hyperplasia in the transplanted patients with coronary vasculopathy. Coronary intravascular ultrasound (IVUS) is a useful and reliable modality for evaluating coronary vasculopathy. Dobutamine stress echocardiography (DSE) is also safe and reliable screening method for coronary vasculopathy. There is an unproven efficacy for percutaneous coronary intervention (PCI) in these patients. Revascularization strategies such as coronary artery bypass grafting (CABG) appear to be difficult due to diffuse nature of disease; hence they have no role.
Statins and diltiazem are advised in an initial transplant as a strategy to reduce the incidence and progression of cardiac allograft vasculopathy (CAV). Retransplantation is the only definitive therapeutic option.
Neoplasms
Malignancies are among the primary causes of late mortality after HTx. Cutaneous squamous cell carcinomas are the most common transplant-related skin cancers.18 Malignant tumors includes non-Hodgkin’s lymphoma and Hodgkin’s lymphoma (as a part of the posttransplant lymphoproliferative disease),19 both linked to viral infection, anogenital cancers (linked to the human papilloma virus), and hepatic cancer (hepatitis B and C virus).
Cardiac transplant recipients pose a great risk due to their higher level of immunosuppression. It is also known that patients with a prior history of cancer have an increased risk of developing posttransplant malignancies. The relationship between HLA-G expression and cancers has been well documented.
Immunosuppression
Transplanted organs are unable to survive as a result of immune-mediated attack. According to the ISHLT registry, the triple regimen including corticosteroid, calcineurin inhibitor, and an antiproliferative agent continues to be routinely used at most services.
Multidrug Regime
The most common regimens include
-
Cyclosporine—calcineurin inhibitor
-
Azathioprine (AZA)—antiproliferative agent
-
Prednisone—steroid
-
Newer developments include
-
Tacrolimus (FK 506)—calcineurin inhibitor
-
MMF—antiproliferative agent
-
Sirolimus
-
ATGAM, RATG
-
OKT3
Corticosteroids
Steroids, among the first immunosuppressive agents used in cardiac transplantation, have remained an important component, used at high doses during the initial phases and in acute rejection episodes during induction. Long-term administration of steroids may result in chronic adrenal suppression and adrenal insufficiency.
Calcineurin Inhibitors
Cyclosporine and tacrolimus are calcineurin inhibitors. Cyclosporine has a variable pattern of bioavailability, narrow therapeutic range, and monitoring serum levels to prevent toxicity. Side effects include nephrotoxicity, systemic hypertension, gingival hyperplasia, and tremors. Tacrolimus is a macrolide. Dose is adjusted based on serum levels. Side effects include nephrotoxicity and neurotoxicity.
Recent studies demonstrated a smaller incidence of rejection with tacrolimus as compared with cyclosporine. If resistant to corticosteroids or persistent rejection, it is advisable to exchange cyclosporine for tacrolimus.
Antiproliferative Agents
AZA and MMF are the antiproliferative agents that selectively inhibit lymphocyte proliferation. Side effects include gastrointestinal symptoms and myelosuppression. AZA is a purine analogue that is first rapidly converted to its active form, 6-mercaptopurine, and is subsequently further converted to thio-inosine-monophosphate. This antimetabolite is incorporated into DNA and inhibits its synthesis, thereby preventing further proliferation of activated T and B lymphocytes. This drug is typically used with a calcineurin inhibitor or glucocorticoids. The major side effects include dose-dependent myelosuppression, particularly leucopenia, skin cancer, cutaneous fungal infections, and, rarely, liver toxicity and pancreatitis.
AZA should be temporarily withheld if the white cell count decreases to 50% compared with the previous value or falls below 3,000/mm3. Studies demonstrated comparison between AZA and mycophenolate and revealed superiority of mycophenolate, in terms of rejection and survival and a possible reduction in coronary artery vasculopathy and neoplasms.
Based on these results, mycophenolate became the antiproliferative agent of choice in HT in association with calcineurin inhibitors and corticosteroids. MMF is also a prodrug that inhibits inosine monophosphate dehydrogenase, a critical enzyme for the de novo purine synthesis by lymphocytes, thus inhibiting their proliferation. Therefore, it selectively inhibits both T- and B-lymphocyte proliferation. MMF is available as a powder for injection, tablet or capsule, and oral suspension (liquid).
MMF is Food and Drug Administration (FDA) approved for rejection prophylaxis in cardiac transplant renal, hepatic recipients. The drug is typically well tolerated. The major side effects include nausea, vomiting, and diarrhea. The toxicity of MMF may be more closely related to the mycophenolic acid levels than the dose. Studies have demonstrated that the risk of opportunistic infections is higher in patients treated with MMF when compared with AZA.
Mechanistic Target of Rapamycin Inhibitors
These are sirolimus and everolimus that inhibit the mechanistic target of rapamycin (mTOR), which blocks the cellular response cytokines and inhibits vascular smooth muscle cell growth and proliferation in response to various growth factors.
Sirolimus, also known as rapamycin, is a macrocyclic lactone produced by Streptomyces hygroscopicus and inhibits T-cell activation and proliferation. To minimize the pharmacokinetic interaction between the two drugs, sirolimus should be given 4 hours after cyclosporine administration.
Sirolimus is available in a tablet or liquid formulation. Dose of sirolimus is adjusted typically to achieve serum trough levels of 5 to 10 ng/mL. It lowers the incidence of acute cellular rejection and slows the progression of transplant vasculopathy.20 Both the drugs demonstrated a reduction in incidence and a progression of CAV.
The most common drug-related side effects include hyperlipidemia with hypertriglyceridemia, thrombocytopenia, neutropenia, increased low-density lipoprotein (LDL) cholesterol, and anemia. Rare but serious adverse effects of sirolimus-related pulmonary toxicity have been described.
Thrombocytopenia seems to be dose related and but is reversible. When given without cyclosporine sirolimus, it does not appear to result in renal dysfunction or diabetes.
Everolimus
Everolimus, an analogue of sirolimus, has not yet been approved for clinical use. The main difference between everolimus and sirolimus is that the half-life of everolimus (30 hours) is approximately half that of sirolimus (60 hours). The preliminary reports from several studies in HTx recipients demonstrate positive results.
Antilymphocyte Globulin
It is a polyclonal antibody designed to inhibit T cells by binding to surface antigens. Goal is to keep T-lymphocyte count approximately 200 cells/µL if the patient has been administered antilymphocyte globulin. Side effects include fevers, chills, urticaria, serum sickness, and thrombocytopenia.
Muromonab-CD3 (OKT3)
Muromonab-CD3 is a murine monoclonal antibody and selectively depletes T cell. It exerts its immunosuppressive effects via a rapid T-cell depletion from the peripheral circulation and modulation of the T-cell receptor-CD3 antigen recognition complex, thereby blocking the immunologic function of these cells.
The major side effects include cytokine release syndrome that is caused by initial activation of T cells and release of multiple cytokines, manifested by fevers, rigors, nausea, vomiting, diarrhea, hypotension, chest pain, dyspnea or wheezing, arthralgias, and myalgias. Other rare life-threatening complications include pulmonary edema, encephalopathy, and aseptic meningitis. CMV infection and posttransplant lymphoproliferative disorders are the long-term adverse reactions. Finally, prolonged use of OKT3 increases the risk of antibody-mediated rejection. The use of OKT3 as induction therapy in HTx has significantly declined between 1997 and 2007 due to these adverse effects and the availability of alternative agents.
Anesthesia for Patients with Previous Transplant
The transplanted heart has no sensory, sympathetic, and parasympathetic innervations, along with loss of vagal tone. Patients with transplanted heart are preload dependent and rely on changes in stroke volume as CO is highly dependent on venous return. Agents that act indirectly via the sympathetic or parasympathetic system (atropine, ephedrine) will be ineffective whereas drugs with a direct/indirect effect will only have their direct effect seen.
Immunosuppressants’ side effects include nephrotoxicity as well as neurotoxicity, and cyclosporine is associated with cholelithiasis, increasing the incidence of cholecystectomy in these patients. A significant number of transplanted patients develop diastolic dysfunction, manifested as exercise intolerance. Abnormalities in isovolumic relaxation time corresponding with varying degrees of rejection, increased peak inflow velocity, and mitral deceleration are indicators of restrictive filling. The presence of rejection causes inflammatory infiltrate that increases perioperative morbidity and the incidence of asymptomatic arrhythmias.
Preoperatively, assess the current function of the transplanted heart. Thoroughly review important laboratory findings such as hematology results, blood biochemistry, and coagulation status. One should look for signs of rejection that can be a possible complication related to immunosuppression that should be considered. Review current electrocardiogram (ECG) that may show a double P wave, reflecting atrial activity in the native atrial cuff and the transplanted atrium.
Other investigations such as stress test, transthoracic echocardiography, cardiology consult, recent cardiac catheterization, and myocardial biopsy should be reported. Examine other organ involvement due to long-term use of immunosuppressant medications. To maintain an optimum blood level and avoid possible organ rejection, immunosuppressants should be continued as scheduled before the surgery. Standard premedication may be used unless contraindicated. All aseptic precautions should be taken to prevent infection. Administer corticosteroid before induction. If the patient is on pacemaker or implanted defibrillator, ensure proper function of the same.
Choice of anesthetic depends on the type of surgery and the patient’s condition. Regional anesthesia can be used cautiously, with the knowledge that these patients cannot respond to vasodilation and hypotension. Cardiovascular monitoring depends on the nature of the planned surgery. Intraoperative echocardiography is important in managing volume status.
Ephedrine or isoproterenol should be readily available to treat bradycardia as atropine will not have an effect. N2O can also worsen preexisting pulmonary hypertension. Immediate postoperative problems include low CO, RV dysfunction, bleeding, posttransplant arrhythmias, renal failure, and rejection.
Postoperative Management
The heart-transplanted patients are complicated in particular by RV dysfunction, which can become life threatening. Therefore, the aim of postoperative therapy is individually tailored to preload conditions and ensure contractility and an adequate reduction in the pulmonary vascular resistance. Mechanical ventilation enables the RV afterload to be specifically influenced and to maintain adequate gas exchange. The two important components of the intensive therapy after HTx are immediate immunosuppressive therapy and rejection monitoring. It is of particular importance that the patient undergoing heterotopic transplantation mandates anticoagulation.
Conclusion
HTx offers a second chance of life to people with end-stage heart failure and has a lot of influence on the transplant patients’ quality of life. Management of donor recipient condition is most important. Right-sided heart failure is the most common cause of postoperative transplant patient. In postoperative period, prolonged poor cardiac function leads to prolong intubation and subsequently leads to fatal infections. Hence postoperative care is the most difficult aspect in HTx.
References
- Advanced chronic heart failure: A position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2007;9:684-694. 6-7
- [Google Scholar]
- 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: A report of the American college of cardiology foundation/American heart association task force on practice guidelines: developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119(14):1977-2016.
- [Google Scholar]
- Selection of cardiac transplantation candidates in 2010. Circulation. 2010;122(2):173-183.
- [Google Scholar]
- Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83(3):778-786.
- [Google Scholar]
- The Heart Failure Survival Score outperforms the peak oxygen consumption for heart transplantation selection in the era of device therapy. J Heart Lung Transplant. 2011;30(3):315-325.
- [Google Scholar]
- The prospective use of plasmapheresis and IVIG in sensitized patients prior to heart transplantation. J Heart Lung Transplant. 2004;23:S61.
- [Google Scholar]
- Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail. 2009;2(4):320-324.
- [Google Scholar]
- Mechanical circulatory support therapy as a bridge to transplant or recovery (new advances) Curr Opin Cardiol. 2006;21(2):120-126.
- [Google Scholar]
- Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10):931-940.
- [Google Scholar]
- Intraaortic balloon pumping for predominantly right ventricular failure after heart transplantation. Ann Thorac Surg. 2000;70(5):1587-1593.
- [Google Scholar]
- Impact of bacterial and fungal donor organ contamination in lung, heart-lung, heart and liver transplantation. Infection. 2008;36(3):207-212.
- [Google Scholar]
- Infectious complications among 620 consecutive heart transplant patients at Stanford University Medical Center. Clin Infect Dis. 2001;33(5):629-640.
- [Google Scholar]
- Fulminant mixed humoral and cellular rejection in a cardiac transplant recipient: a review of the histologic findings and literature. J Heart Lung Transplant. 2003;22(5):604-607.
- [Google Scholar]
- Pretransplantation risk factors for acute rejection after heart transplantation: a multiinstitutional study. J Heart Lung Transplant. 1993;12(3):355-366.
- [Google Scholar]
- A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. J Heart Transplant. 1990;9(6):587-593.
- [Google Scholar]
- Incidence and treatment of neoplasia after transplantation. J Heart Lung Transplant. 1993;12:S328-S336. 6 Pt 2 suppl
- [Google Scholar]
- Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4(2):222-230.
- [Google Scholar]
- Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized clinical trial. Circulation. 2004;110(17):2694-2700.
- [Google Scholar]






