Translate this page into:
Transesophageal Echocardiographic Assessment of Single Ventricle Physiology with Interrupted Inferior Vena Cava in a Case of Heterotaxy Syndrome Posted for Kawashima Procedure and Hepatic Vein Rerouting
*Corresponding author: Neeti Makhija, Department of Cardiac Anaesthesia and Critical Care, All India Institute of Medical Sciences, New Delhi, Delhi, India. neetimakhija@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Das D, Makhija N, Prakash M. Transesophageal Echocardiographic Assessment of Single Ventricle Physiology with Interrupted Inferior Vena Cava in a Case of Heterotaxy Syndrome Posted for Kawashima Procedure and Hepatic Vein Rerouting. J Card Crit Care TSS. doi:10.25259/JCCC_53_2023
Abstract
Transesophageal echocardiography (TEE) is essential in delineating the anatomy, physiology, and perioperative management of single ventricle (SV) lesions. SV lesion is commonly associated with heterotaxy syndrome causing abnormal lateralization of organs across the body’s left-right axis. It is manifested as right atrial or left atrial isomerism. Patients with SV physiology require the Glenn procedure for surgical palliation whereas Fontan operation as definitive intervention. However, in a patient with interrupted inferior vena cava (IVC), the Kawashima procedure is used for definitive palliation. We report a 15-year-old female child diagnosed with SV physiology and interrupted IVC in association with heterotaxy syndrome posted for the Kawashima procedure and hepatic vein rerouting and illustrate the importance of perioperative TEE in delineating the anatomy, decision-making, and confirming the success of repair.
Keywords
Heterotaxy syndrome
Interrupted inferior vena cava
Kawashima procedure
Single ventricle physiology
Transesophageal echocardiography
INTRODUCTION
Single ventricle (SV) physiology is commonly associated with heterotaxy syndrome occurring in approximately 1 in 10,000 live births and present in approximately 3% of congenital heart disease cases.[1-3] It is manifested as right atrial isomerism (two similar right atria and duplication of right-sided organs) and left atrial isomerism (two similar left atria and duplication of left-sided organs).
Conventionally, echocardiography, especially the transthoracic approach, has been the first-line diagnostic modality. Other imaging techniques include computed tomography (CT) angiography, and magnetic resonance imaging. Intraoperatively, transesophageal echocardiography (TEE) plays a vital role in confirming the diagnosis, guiding the surgical technique, and determining the success of surgery.
We report a 15-year-old female child, who is a diagnosed case of SV physiology and interrupted inferior vena cava (IVC) with heterotaxy syndrome and left atrial isomerism, was scheduled for Kawashima procedure and hepatic vein (HV) rerouting and the importance of perioperative TEE in delineating the anatomy, decision-making, and confirming the success of repair.
CASE REPORT
A 15-year-old female child was admitted with shortness of breath, easy fatigability and bluish discoloration of lips and nails. The child had cyanosis and clubbing with a room air saturation of 75%. Transthoracic echocardiography demonstrated SV physiology with double outlet right ventricle, large ventricular septal defect (VSD), hypoplastic mitral valve, and severe pulmonary stenosis (PS). CT angiography confirmed polysplenia, left isomerism of bronchial situs, interrupted IVC with azygos continuation, HVs forming suprahepatic IVC, large atrial septal defect, dilated right ventricle, large subaortic VSD, and infundibular and valvular PS.
With the diagnosis of SV physiology with heterotaxy syndrome and interrupted IVC, the surgical plan was for the Kawashima procedure and HV rerouting. Tablet metoprolol was continued till the morning of surgery. Standard ASA monitors were attached to the patient in the operating theater. Large bore peripheral venous access was secured. Balanced anesthesia technique was used for induction, and the airway was secured. Central venous access and arterial access are secured in the femoral vessels. For superior vena cava (SVC) pressure monitoring, access was taken in the right internal jugular vein.
TEE was performed using an X7-2t Philips ultrasound probe and machine (iE33 model; Philips, Bothell, WA, USA). Midesophageal 4-chamber view demonstrated predominant right cardiac chambers with a small left-sided a trium a nd hypoplastic mitral valve [Figure 1a]. Focusing on the right cardiac chambers, the morphology of the right atrial appendage was alike the left a trial a ppendage [ Figure 1 b]. R otating t he probe to the left side showed the left pulmonary vein draining to the right-sided atrium [Figure 1c] and confirmed b y p ulse wave Doppler pattern [Figure 1d]. SVC and IVC were found to be draining into the right-sided atrium in the mid-esophageal bicaval view [Figure 2a and b]. On further insertion of the probe, three HVs combined to form the supra-hepatic portion of IVC [Figure 2c and d, Video 1]. Kawashima procedure with HV rerouting was done with Gelweave Y graft ( 18 × 9 m m). The graft was connected from the HV confluence to the right and left pulmonary arteries (PAs). The child was weaned after 260 min of bypass and 98 min of aortic cross-clamp with sodium nitroprusside 0.5, dobutamine 5 mcg/kg/min, and vasopressin 1 unit/h. Postoperatively the graft p atency [ Figure 3a] w as confirmed, and the proximal anastomotic site to HV confluence [Figure 3b] and distal anastomotic site of Y graft to the respective PAs [Figures 3c and d] were found unobstructed.
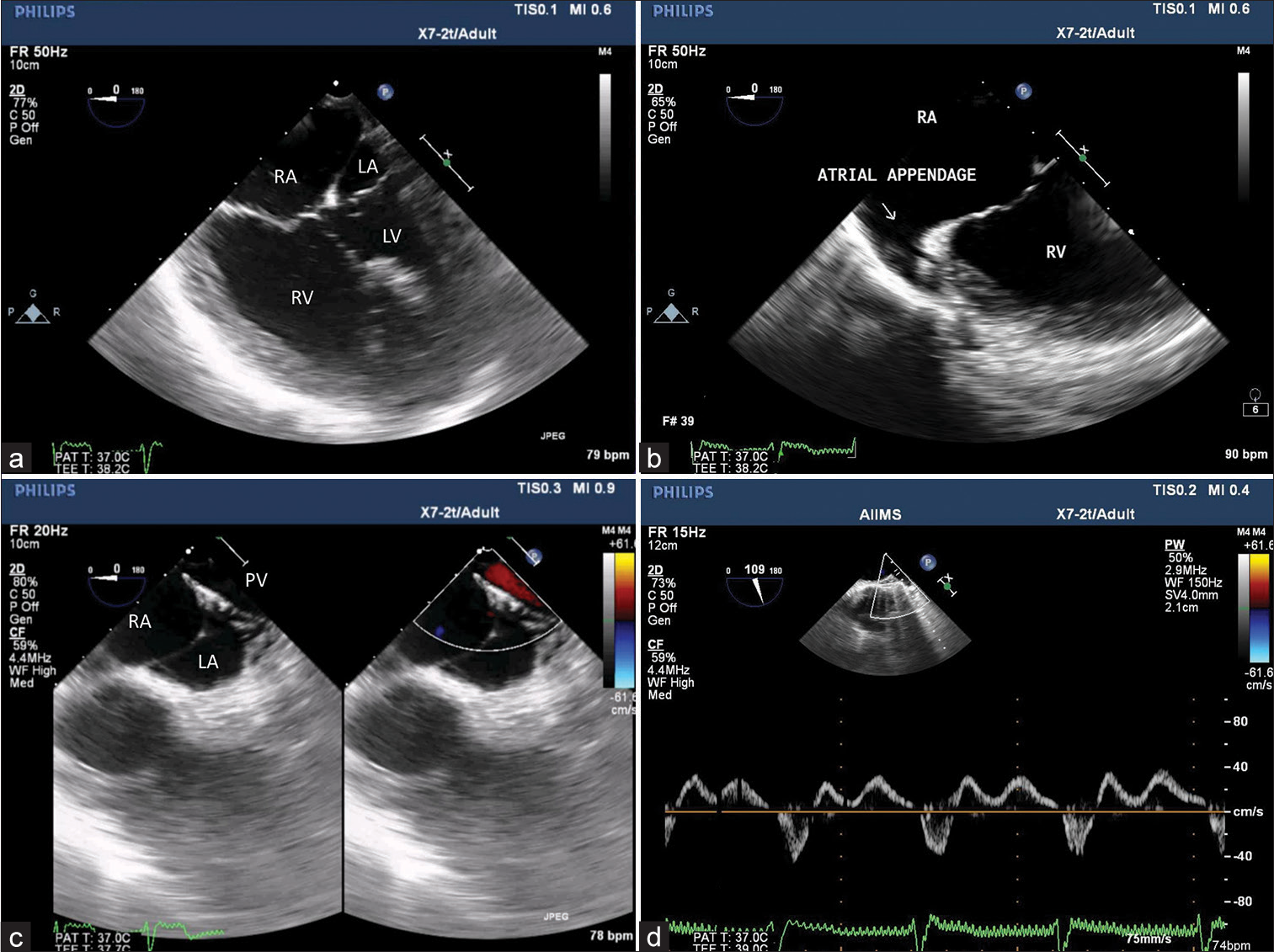
- (a) Midesophageal 4-chamber view showing dominant right-sided atrium (RA) and ventricle whereas hypoplastic left-sided atrium (LA) and ventricle (LV). (b) Tilting to the right side demonstrates left isomerism of right-sided atrial appendage. (c) Further focusing, the left upper pulmonary vein (PV) draining to RA. (d) Pulse wave Doppler interrogation characterizes PV waveform.
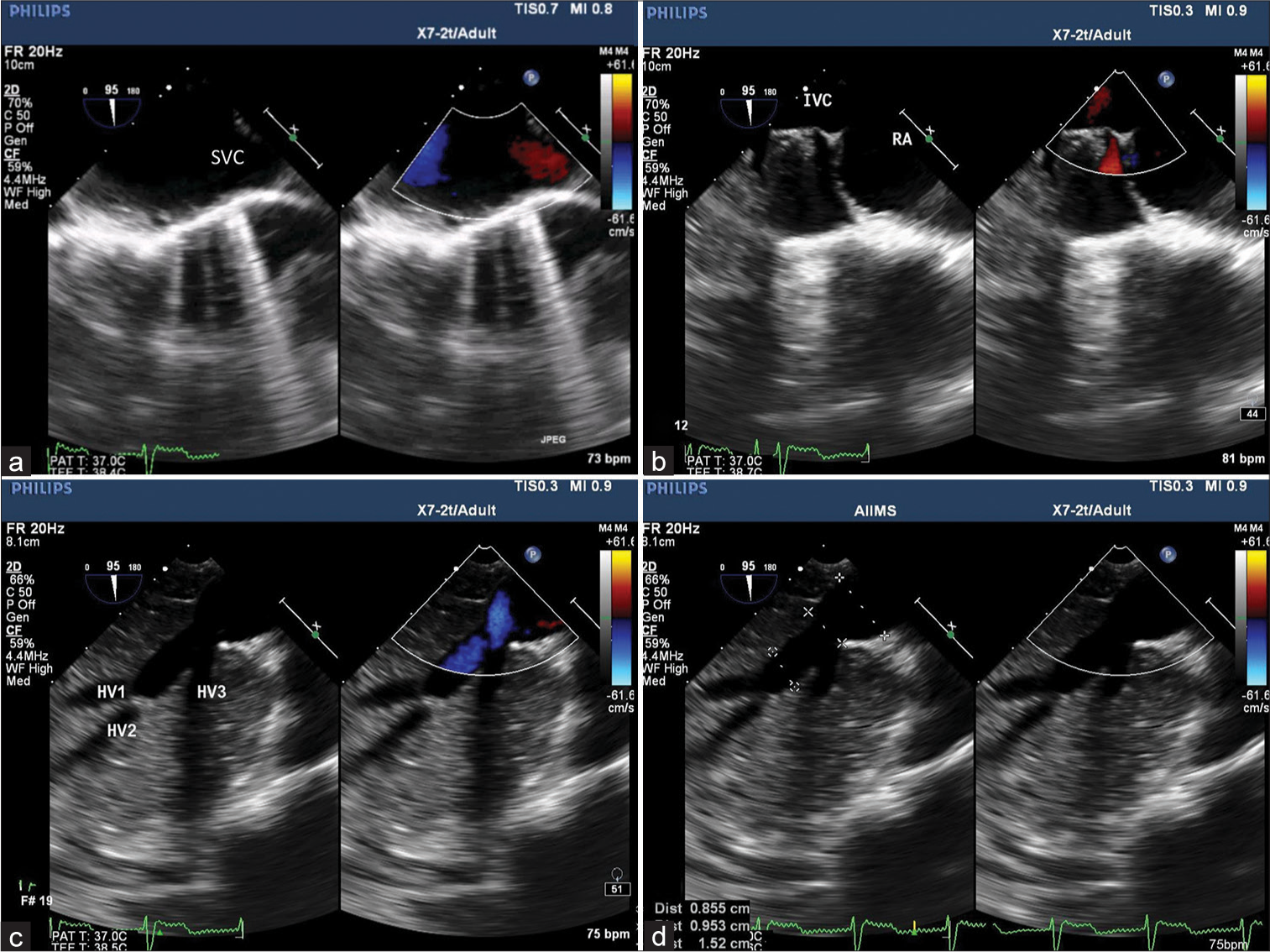
- (a) Midesophageal bicaval view with color comparison demonstrating superior vena cava and (b) inferior vena cava (IVC) draining into the right atrium. (c) Transgastric hepatic vein (HV) view confirming 3 HV draining into IVC and (d) HV confluence and IVC measuring 0.9 cm and 1.5 cm, respectively.

- (a) Midesophageal view focusing on the graft illustrates its patency, (b) proximal anastomotic site to supra-hepatic inferior vena cava, and orthogonal image (b1 and b2) showing patent distal anastomotic site of Y graft corresponding to the (c and d) respective pulmonary arteries.
Video 1:
Video 1:Transgastric hepatic vein (HV) view demonstrating interrupted inferior vena cava (IVC) and 3 HV draining into suprahepatic IVC.After surgery, the child was shifted to the intensive care unit, where the patient was extubated after four hours and was discharged after eight days of hospital stay.
DISCUSSION
In the left atrial isomerism, the morphology of both atrial appendages is tubular.[4] Venous malformations may include bilateral SVC. However, the majority of patients have an interrupted IVC, with absent suprarenal IVC segment and azygos or hemiazygos continuation to the SVC. Finally, bilateral common HVs usually drain either to the right or left atrium.
Cardiac surgical intervention for patients with heterotaxy syndrome depends on ventricular physiology and circulatory balance. For patients with left atrial isomerism having two working ventricles, a definitive biventricular repair is sometimes possible. For patients with SV physiology, a sequence of palliative surgeries is required to prepare for the Fontan procedure. When the cardiac lesions prevent adequate pulmonary circulation, a systemic-to-PA shunt is often the first palliative step. Alternatively, if pulmonary blood flow is in excess, a PA band can be placed to maintain circulatory balance.[4] Left atrial isomerism is usually associated with pulmonary overcirculation rather than inadequate pulmonary blood flow. Because a systemic-to-PA is meant to redirect more blood flow to the lungs, this intervention is not appropriate for our patient with left atrial isomerism. Usually, 2–3 months after, once pulmonary vascular resistance has dropped, a bidirectional Glenn shunt can be made to direct partial systemic venous drainage directly to the PAs.[5] For patients with left atrial isomerism who have an interrupted IVC with azygos continuation, a greater portion of the systemic venous return is directed through the SVC, therefore, a Kawashima procedure can be performed for low operative risk and acceptable long-term surgical outcome.[6] This procedure, first described by Kawashima in 1984, creates an end-to-side anastomosis between each SVC and the ipsilateral PA and ligates or partially ligates the main PA.[7] In our case, the child had heterotaxy syndrome with left atrial isomerism, interrupted IVC (suprarenal), azygos continuation of IVC, and HVs combined to form a common trunk that directly drains to the right-sided atrium. The 15-year-old child had SV physiology with adequate size PA, therefore the plan was Kawashima procedure with HV rerouting. Cavopulmonary incorporation of HV will reduce hypoxemia due to a combination of resolution of pulmonary arteriovenous malformation and elimination of hepatic venoatrial right-to-left shunting.[8,9]
TEE played an important role in confirming the diagnosis, delineating the venous drainage to respective cardiac chambers, clear illustration of HVs forming the common trunk and determining surgical adequacy in the form of patency of graft, absence of flow turbulence at anastomotic site, unobstructed venous drainage, and appropriate rerouting of HVs postoperatively. From preoperative delineation of complex congenital anomaly, intraoperative surgical guidance to successful postoperative surgical repair, TEE remains an inevitable tool in each step of the perioperative course. Therefore, it will be difficult to guide the perioperative course in the absence of TEE, especially in this evidence-based management era.
CONCLUSION
Heterotaxy syndrome with SV physiology and interrupted IVC forms complex congenital malformation. Surgical repair and anesthetic management were challenging. The requirement of TEE is critical in every step during the perioperative period, beginning from preoperative confirmation of diagnosis, surgical decision-making, ventricular function, and ensuring adequate postoperative repair.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Videos available on:
Financial support and sponsorship
Nil.
References
- Predictors of Poor Outcome among Children with Heterotaxy Syndrome: A Retrospective Review. Open Heart. 2016;3:e000328.
- [CrossRef] [PubMed] [Google Scholar]
- Fetal Situs, Isomerism, Heterotaxy Syndrome: Diagnostic Evaluation and Implication for Postnatal Management. Curr Treat Options Cardiovasc Med. 2016;18:77.
- [CrossRef] [PubMed] [Google Scholar]
- Heterotaxy Syndrome: Proceedings From the 10th International PCICS Meeting. World J Pediatr Congenit Heart Surg. 2015;6:616-29.
- [CrossRef] [PubMed] [Google Scholar]
- Cyanotic Congenital Heart Defects In: Pediatric Cardiology for Practitioners (6th ed). Philadelphia, PA: Elsevier Health Sciences; 2014. p. :206-89.
- [CrossRef] [Google Scholar]
- Outcomes of Multistage Palliation of Infants with Functional Single Ventricle and Heterotaxy Syndrome. J Thorac Cardiovasc Surg. 2016;151:1369-77.e2.
- [CrossRef] [PubMed] [Google Scholar]
- Kawashima Procedure: The Impact of Age. Ann Thorac Surg. 2023;116:366-72.
- [CrossRef] [PubMed] [Google Scholar]
- Total Cavopulmonary Shunt Operation in Complex Cardiac Anomalies. A New Operation. J Thorac Cardiovasc Surg. 1984;87:74-81.
- [CrossRef] [PubMed] [Google Scholar]
- Incorporation of the Hepatic Veins into the Cavopulmonary Circulation in Patients with Heterotaxy and Pulmonary Arteriovenous Malformations after a Kawashima Procedure. Ann Thorac Surg. 2005;80:1597-603.
- [CrossRef] [PubMed] [Google Scholar]
- Extracardiac Fontan Completion after the Kawashima Procedure with a Custom-made Bifurcated Graft. Multimed Man Cardiothorac Surg. 2022;2022
- [Google Scholar]







