Translate this page into:
Technical Details of Aortic Valve Replacement using Carpentier–Edwards PERIMOUNT Magna Ease Aortic Bioprosthesis in a Sexagenarian Patient with Severe Calcific Aortic Stenosis: A Video Presentation
Dr. Ujjwal Kumar Chowdhury, MCh, Diplomate NB Department of Cardiothoracic and Vascular Surgery, All India Institute of Medical Sciences New Delhi-110029 India ujjwalchow@rediffmail.com ujjwalchowdhury@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Introduction
The current guidelines of the American Heart Association (AHA), American College of Cardiology (ACC) from 2014, and the European Society of Cardiology (ESC) from 2012 uniformly recommend mechanical aortic valve replacement in patients under 60 years of age and biologic aortic valve replacement in patients over 70 years of age.1, 2 The recommendations are conflicting for patients between 60 and 70 years of age. The ESC guidelines recommend biologic prosthesis from the age of 65 years onward, whereas the newer AHA/ACC guidelines only recommend biological valves starting 70 years of age. Over the past 20 years, there is a shift away from a clear-cut age limit toward patient’s wish and lifestyle considerations.3
The number of surgical aortic valve replacements using a bioprosthesis is increasing according to the annual surveys of thoracic surgery in 2013 and 2014 by the Japanese Association for Thoracic Surgery, which states that bioprostheses are used in three-quarters of all aortic valve replacements procedures.4 In addition, the age limit for implantation of an aortic bioprosthesis is continuously being shifted down with bioprostheses used for aortic valve replacements in 60% of sexagenarian patients and 90% of septuagenarian or octogenarian patients.3, 4, 5, 6 This may be related to the enhanced durability of new-generation bioprostheses, improved outcomes of redo valve replacement surgery, or the development of valve-in-valve (ViV) transcatheter aortic valve implantation.3, 4, 5, 6
Randomized trials comparing biological and mechanical valve replacements are scanty. In 2009, Stassano and associates randomized 310 patients between 55 and 70 years of age into a mechanical and a biological prosthesis group to undergo aortic valve replacement. At a mean follow-up of 4 years, they found similar mortality and other adverse prosthesis-related events, namely, thromboembolic complications, bleeding, endocarditis, and structural valvular deterioration in the two group of patients.7
In 2008, Brown and associates analyzed outcome after aortic valve replacement with mechanical versus biological prosthesis in patients aged between 50 and 70 years at operation. Freedom from reoperation was 98% for mechanical valve and 91% for bioprosthesis (p = 0.06). Rehospitalization for hemorrhagic events occurred in 15% of patients with mechanical valves and 7% of patients with bioprosthesis (p = 0.001). The 5- and 10-year unadjusted survivals were 87% and 68% for mechanical valves and 72% and 50% for bioprosthesis, respectively.8 The reported incidence of survival following mechanical mitral valve replacement in the published literature at 10, 20, and 30 years was 61 to 75%, 36.5 to 39% and 22.6%, respectively.9, 10, 11, 12, 13, 14
The Carpentier–Edwards pericardial prosthesis commercially available since 1980 is the bioprosthesis, which is the most used worldwide. As a second-generation pericardial bioprosthesis, the Carpentier–Edwards pericardial valve was designed to minimize structural valvular deterioration, which plagued the first-generation prosthesis while retaining the hemodynamic superiority conferred by pericardial valve substitutes.14, 15, 16, 17 Published literature documents excellent long-term outcomes with the Carpentier–Edwards pericardial valve (Table 1).15, 18, 19, 20, 21, 22
|
Model |
Author |
Follow-up maximum, mean (years) |
Time of structural valve deterioration estimate (years) |
Age (years) |
Freedom from structural valve deterioration estimate (%) |
|---|---|---|---|---|---|
|
Abbreviations: CI, confidence interval. |
|||||
|
Carpentier–Edwards |
Poirier et al15 |
15, 4.8 |
14 |
Mean (not reported) < 60 60–69 ≥70 |
79.9 ± 5.0 84.7 87.9 100 |
|
Carpentier–Edwards |
Neville et al18 |
12, 4.7 |
12 |
Mean 68 < 60 ≥60 |
94 (CI: 90–98) 89 (CI: 80–98) 98 (CI: 96–100) |
|
Carpentier–Edwards |
Banbury et al19 |
17, 12 |
15 |
Mean 65 < 50 50–70 ≥70 |
77 (CI: 74–82) 48 80 90 |
|
Carpentier–Edwards |
Dellgren et al22 |
14, 5 |
12 |
Mean 71 > 65 |
86 ± 9.0 100 |
|
Carpentier–Edwards |
Biglioli et al20 |
18, 6.0 |
18 |
Mean 67 < 65 ≥65 |
52.9 ± 9.9 35.8 ± 10.7 83.7 ± 8.9 |
|
Carpentier–Edwards |
McClure et al21 |
17, 6.0 |
15 |
Mean 74 < 65 65–75 ≥75 |
82.3 (CI: 67–91) 34.7 CI: 6–67) 99.5 (CI: 97–99.9) 99.5 (CI: 97–99.0) |
The PERIMOUNT Magna Ease which is a further development of the PERIMOUNT Magna prosthesis (Edwards Lifesciences, Irvine, CA, USA) belongs to the latest generation of aortic valve prostheses. The manufacturers points the following advantages: lower profile, contoured and complaint sewing rings, and larger effective orifice areas, which would result in easier insertion and higher coronary ostial clearance; further, it lowers transprosthetic gradients and avoids prosthesis-patient mismatch; also, lower gradients together with an anticalcification technology prevents early structural valve deterioration.23, 24, 25
Although the Carpentier–Edwards PERIMOUNT Magna Ease valve is a bioprosthesis with documented excellent hemodynamics and other advantages as stated above, the valve has a gap between the cobalt-chromium-nickel alloy stent and silicone sewing ring. This gap, which is widest just below each of the three commissural struts, lacks silicone and leaves the two-layer polytetrafluoroethylene fabric unprotected. The passage of a needle through this weak area may result in fabric tear, resulting in a true cuff leakage and not the usual paravalvular leakage. To date, there are four case reports of cuff leakage in the literature diagnosed by transesophageal echocardiography. Three patients with cuff leakage were managed conservatively, and in one patient, it was sutured using polypropylene suture.26, 27, 28, 29 Pledgeted mattress sutures like we have used in our case in this manuscript have been advocated by other authors to prevent this complication.29
We report herein a 61-year-old male patient (body surface area 1.7 m2) diagnosed with severe calcific aortic stenosis, with a peak systolic left ventricle-to-aortic gradient of 110 mm Hg and normal coronaries undergoing aortic valve replacement using a 21 mm Carpentier–Edwards PERIMOUNT Magna Ease aortic prosthesis under moderately hypothermic cardiopulmonary bypass, St. Thomas (II)-based cold blood cardioplegia and iced normal saline. Postoperative recovery was uneventful.
Surgical Techniques
Following median sternotomy, vertical pericardiotomy, and systemic heparinization, cardiopulmonary bypass was established using aorto-atrial cannulation. It was not necessary to dissect the ascending aorta from the main pulmonary artery.
Under cardiopulmonary bypass on a partially filled heart with the ventilation stopped, the left atrial sump suction vent was inserted through the right superior pulmonary vein. (Video 1).
After aortic cross-clamping, an oblique horseshoe-shaped aortotomy was performed in between stay sutures 1.5 cm above the sinus of the right coronary artery, stopping approximately 1 cm above the midpoint of the noncoronary sinus. Myocardial protection was achieved by integrated myocardial protection using direct ostial St. Thomas (II)-based cold blood cardioplegia (4:1) and topical cardiac cooling using ice cold saline. Successive doses of cardioplegia were repeated every 30 minutes.
Three commissural stay sutures were placed on the three aortic commissures. The stenosed calcific aortic valve was excised in a systematic manner, starting at the midpoint of the right coronary cusp and proceeding in an anticlockwise fashion.
The calcified aortic cusp was crushed using a thick hemostat, and the aortic valve was carefully excised, avoiding left ventricle-to-aortic discontinuity and injury to the aorto-mitral curtain.
The left ventricular cavity was thoroughly irrigated using cold normal saline, ensuring no embolization of the calcific debris to the left ventricular cavity and the coronary ostia. The aortic valve was sized and a 21 mm Carpentier–Edwards PERIMOUNT Magna Ease valve was selected for aortic valve implantation. The valve was implanted in an intra-annular location using interrupted, nonpledgeted 2–0 Ticron suture (M/s Covidien Domingo, Dominican Republic, USA) (Figs. 1A H). The aortotomy was closed in two layers using 4–0 polypropylene sutures (Johnson and Johnson Ltd., Ethicon, LLC, San Lorenzo, USA) (Video 1).
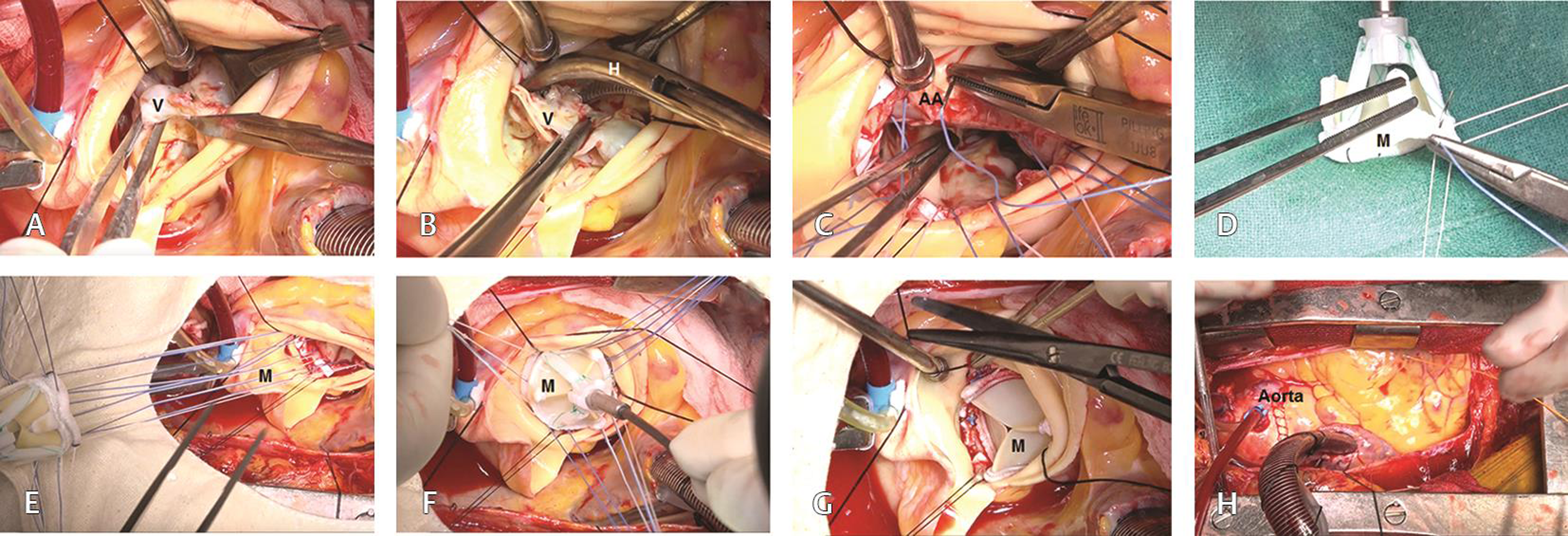
- (A–H) Step-by-step demonstration of implantation of a Carpentier–Edwards Magna Ease aortic bioprosthesis. Abbreviations: AV, calcified aortic valve; H, hemostat; M, Magna Ease bioprosthesis.
Results
The patient was weaned off cardiopulmonary bypass on dopamine 5 µg/kg/min and nitroglycerine 0.5 µg/kg/min. He was extubated after 6 hours. At 6 months follow-up, he is in New York Heart Association functional class I with left ventricular ejection fraction of 0.60 in normal sinus rhythm. Echocardiographically, the mean systolic left ventricle-to-aortic pressure gradient was 8 mm Hg, with no aortic regurgitation and no paravalvular/cuff leakage.
Conclusion
Use of interrupted pledgeted mattress sutures for implantation of Carpentier–Edwards PERIMOUNT Magna Ease aortic bioprosthesis avoids fabric tear in the structurally weak area of the sewing ring of the Magna Ease bioprosthesis, thus minimizing the chances of cuff leakage.
Implantation of the 21 mm Magna Ease prosthesis yielded acceptable results in terms of survival, physical capacity, and hemodynamic behavior during 6 months follow-up period. Periodic evaluation of late clinical and hemodynamic outcomes with regard to the Magna Ease prosthesis, as well as the effect of prosthesis-patient mismatch, if any, on long-term outcomes is needed.
Conflicts of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of the article.
Funding The authors received no financial support for the research, authorship, and/or publication of this article.
References
- AHA/ACC guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:521-643.
- [Google Scholar]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33(19):2451-2496.
- [Google Scholar]
- Tissue valve is the preferred option for patients aged 60 and older. Circulation. 2013;128(12):1365-1371.
- [Google Scholar]
- Current status of cardiovascular surgery in Japan, 2013 and 2014: A report based on the Japan Cardiovascular Surgery Database. 4. Valvular heart surgery. Gen Thorac Cardiovasc Surg. 2018;66(1):13-18.
- [Google Scholar]
- Mechanical versus biological aortic valve replacement strategies. Expert Rev Cardiovasc Ther. 2016;14(4):423-30.
- [Google Scholar]
- SIRIO-TAVI group. Comparative performance of transcatheter aortic valve-in-valve implantation versus conventional surgical redo aortic valve replacement in patients with degenerated aortic valve bioprostheses: systematic review and meta-analysis. Eur J Cardiothorac Surg. 2018;53(3):495-504.
- [Google Scholar]
- Aortic valve replacement: a prospective randomized evaluation of mechanical versus biological valves in patients ages 55 to 70 years. J Am Coll Cardiol. 2009;54(20):1862-1868.
- [Google Scholar]
- Aortic valve replacement in patients aged 50 to 70 years: improved outcome with mechanical versus biologic prostheses. J Thorac Cardiovasc Surg. 2008;135(4):878-884, discussion 884.
- [Google Scholar]
- Valve replacement in the young patient with rheumatic heart disease. Review of a twenty-year experience. J Thorac Cardiovasc Surg. 1990;99(4):631-638.
- [Google Scholar]
- Twenty-year experience with the St Jude Medical mechanical valve prosthesis. J Thorac Cardiovasc Surg. 2003;126(6):2022-2031.
- [Google Scholar]
- Thirty-year results of Starr-Edwards prostheses in the aortic and mitral position. Ann Thorac Surg. 1997;63(3):613-619.
- [Google Scholar]
- Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89(2):635-641.
- [Google Scholar]
- Manikalaivani. Thromboembolic and bleeding complications following St. Jude Medical valve replacement. Annals of Short Reports. 2018;1:1009.
- [Google Scholar]
- Mitral valve replacement using Carpentier-Edwards pericardial bioprosthesis in patients with rheumatic heart disease aged below 40 years: 17-year results. Heart Lung Circ. 2018;27(7):864-871.
- [Google Scholar]
- 15-year experience with the Carpentier-Edwards pericardial bioprosthesis. Ann Thorac Surg. 1998;66(06):S57-S61.
- [Google Scholar]
- Patterns of failure in Ionescu-Shiley bovine pericardial bioprosthetic valves. J Thorac Cardiovasc Surg. 1987;93(6):925-933.
- [Google Scholar]
- Pericardial heterografts: why do these valves fail? J Thorac Cardiovasc Surg. 1988;95(4):577-585.
- [Google Scholar]
- Carpentier-Edwards pericardial bioprosthesis in aortic or mitral position: a 12-year experience. Ann Thorac Surg. 1998;66(06):S143-S147.
- [Google Scholar]
- Age and valve size effect on the long-term durability of the Carpentier-Edwards aortic pericardial bioprosthesis. Ann Thorac Surg. 2001;72(3):753-757.
- [Google Scholar]
- Long-term outcomes of the Carpentier-Edwards pericardial valve prosthesis in the aortic position: effect of patient age. J Heart Valve Dis. 2004;13(01):S49-S51.
- [Google Scholar]
- Late outcomes for aortic valve replacement with the Carpentier-Edwards pericardial bioprosthesis: up to 17-year follow-up in 1,000 patients. Ann Thorac Surg. 2010;89(5):1410-1416.
- [Google Scholar]
- Late hemodynamic and clinical outcomes of aortic valve replacement with the Carpentier-Edwards Perimount pericardial bioprosthesis. J Thorac Cardiovasc Surg. 2002;124(1):146-154.
- [Google Scholar]
- Absence of prosthesis-patient mismatch with the new generation of Edwards stented aortic bioprosthesis. Interact Cardiovasc Thorac Surg. 2010;10(6):884-887, discussion 887–888.
- [Google Scholar]
- The new St Jude Trifecta versus Carpentier-Edwards Perimount Magna and Magna Ease aortic bioprosthesis: is there a hemodynamic superiority? J Thorac Cardiovasc Surg. 2014;147(5):1553-1560.
- [Google Scholar]
- The fluid dynamical performance of the Carpentier-Edwards PERIMOUNT Magna Ease prosthesis. BioMed Res Int. 2018;2018:5429594.
- [Google Scholar]
- Mild nonpara-/nontransvalvular leakage of a stented porcine valve implanted in the mitral position decreased after the administration of protamine and disappeared after surgery. J Cardiothorac Vasc Anesth. 2008;22(5):799-800.
- [Google Scholar]
- Transvalvular leakage after the implantation of stented bovine pericardial valves is not only central. J Anesth. 2009;23(4):639-640.
- [Google Scholar]
- Regurgitant leak from the area between the stent post and the sewing ring of a stented bovine pericardial valve implanted in the aortic valve position. Cardiovasc Ultrasound. 2010;8:52.
- [Google Scholar]
- Cuff leakage, not paravalvular leakage, in the Carpentier Edwards PERIMOUNT Magna Ease aortic bioprosthesis. Interact Cardiovasc Thorac Surg. 2015;21(6):796-797.
- [Google Scholar]






