Translate this page into:
ROTEM and Bivalirudin: The Confounding Challenges Ahead
*Corresponding author: Omer Mohammed Mujahid, Senior Resident, Department of Cardiac Anaesthesiology and Critical Care, Cardiac Thoracic Centre, All India Institute of Medical Sciences, New Delhi, India. omermohd1992@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mujahid OM, Prakash M, Choudhury M. ROTEM and bivalirudin: The confounding challenges ahead. J Card Crit Care TSS 2023;7:71-4.
Abstract
Direct thrombin inhibitors directly bind to active sites on thrombin and exert their anticoagulant effect. Bivalirudin is used an alternative in cases of heparin-induced thrombocytopenia, heparin resistance, anaphylactic reaction to UFH, or anti-thrombin III deficiency. Here, we report a case of a 31-year-old female patient, diagnosed with recurrent chronic thromboembolic pulmonary hypertension posted for redo pulmonary thromboendarterectomy. Monitoring anticoagulation during bivalirudin therapy is sometimes confounding and challenging. Prolongation of CT on INTEM and HEPTEM has shown moderate to strong correlation with traditional laboratory tests like aPTT and HPTT (aPTT with Hepzyme). ROTEM can be a way forward for monitoring bivalirudin therapy.
Keywords
ROTEM
Bivalirudin
Direct Thrombin Inhibitor
INTRODUCTION
Bivalirudin is a direct thrombin inhibitor, which is being used as an alternative for anticoagulation in patients on extracorporeal circulation. Direct thrombin inhibitors directly bind to active sites on thrombin and exert their anticoagulant effect. Bivalirudin is used an alternative in cases of heparin-induced thrombocytopenia, heparin resistance, anaphylactic reaction to UFH, or anti-thrombin III deficiency.[1] Bivalirudin has a half-life of 24 min, and unlike heparin, it cannot be reversed as there is no antidote. A major drawback of using bivalirudin is the difficulty in monitoring its anticoagulation effect. ACT and activated partial thromboplastin time (aPTT) are used to monitor anticoagulation with bivalirudin. The aPTT and ACT are both elevated in bivalirudin therapy. Over-coagulation as well as under-coagulation has consequences of bleeding and thrombosis. Ecarin testing is the gold standard for monitoring bivalirudin anticoagulation but not easily available. ROTEM parameters are point of care testing equipment. Viscoelastic testing provides a way forward to diagnose and monitor the anticoagulation in patients on bivalirudin therapy.
CASE REPORT
Here, we report a case of a 31-year-old female patient, diagnosed with recurrent chronic thromboembolic pulmonary hypertension posted for redopulmonary thromboendarterectomy. She is a known case of systemic lupus erythematosus and an underlying anti-phospholipid antibody syndrome, and history of heparin resistance. She had undergone pulmonary thromboendarterectomy 9-years-ago, after which she was diagnosed with heparin resistance. She underwent redopulmonary thromboendarterectomy at our center and at the end of the procedure, she was put on extracorporeal membrane oxygenation (ECMO) support. Bivalirudin was used for anti-coagulation on cardiopulmonary bypass (CPB) and on ECMO. ACT and other hematological laboratory parameters were used to monitor anticoagulation in the perioperative period [Table 1]. Patient as on November 12, 2022, has a tracheostomy and is on inotropic infusions of Adrenaline at 0.1 mcg/kg/min, Noradrenaline at 0.2 mcg/ kg/min, Vasopressin at 1 unit/h, and currently doing well. Viscoelastic testing using ROTEM was done in the post-operative period when the patient was on ECMO. Sample was collected in a citrate containing tube and tests performed. We discussed the ROTEM finding while on ECMO with bivalrudin infusion.
| Parameters | October 18, 2022 (On ECMO bivalirudin) | November 12, 2022 (No ECMO bivalirudin) |
|---|---|---|
| aPTT (s) | 95.6 | 67.4 |
| ACT(s) | 243 | 190 |
| Platelet count (×103/mm3) | 73 | 112 |
| Hb (g/dL) | 10.9/33.6 | 8.9/27.9 |
aPTT: Activated partial thromboplastin time, ECMO: Extracorporeal membrane oxygenation, ACT: Activated clotting time
The patient was on bivalirudin during and post-surgery while on ECMO. The anticoagulation profile of this patient from laboratory parameters is as enumerated in Table 1.
ROTEM analysis
Point of care testing was done using ROTEM while on bivalirudin infusion. On ROTEM, using all four channels, EXTEM, INTEM, HEPTEM, and FIBTEM were performed on the samples with results as shown in [Figure 1]. EXTEM revealed a prolonged CT of 173s, CFT of 361s with a decreased A5 and A10 (19 mm and 24 mm, respectively). The high CT implies a high bleeding risk with possible defects in factors I, II, V, VII, and X and platelets function [Figure 1].

- EXTEM parameters showed a prolonged CT of 173s, CFT of 361s and decreased A5, A10, and A20 parameters.
The decreased A5 and A10 in EXTEM suggest thrombocytopenia and as reported by Parastatidou et al., these two were strong predictors of hemorrhage as compared to most other ROTEM variables, quantifying clot elasticity and platelet components in thrombocytopenia in these critically ill patients.[2,3]
Comprehensive interpretation of the analysis reveals a prolonged CT on EXTEM (173s) and a normal EXTEM A5 (19 mm) along with a normal FIBTEM A5 (15 mm) which ultimately points to a thrombocytopenia [Figures 2 and 3]. A EXTEM A10 cutoff <37 mm (sensitivity = 91% and specificity of 76%) has been demonstrated by most authors as the best prognostic performance parameters for prediction of bleeding events in thrombocytopenic patients.[4]
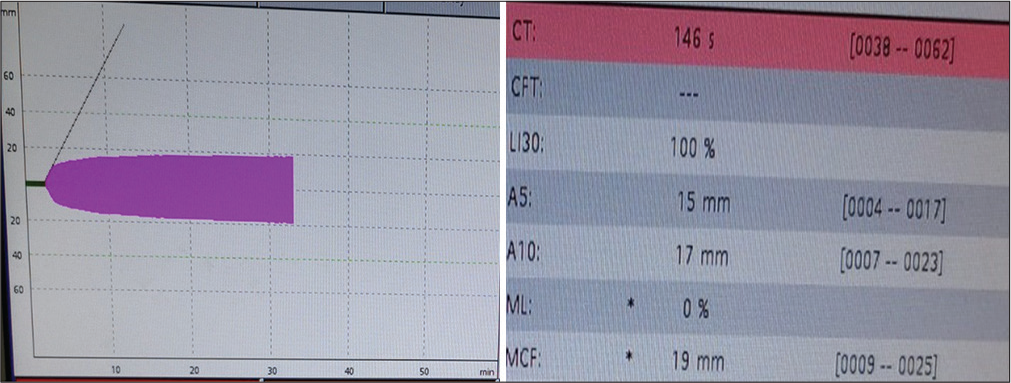
- The FIBTEM analysis showed a prolonged CT of 146s with a normal A5 and A10 of 15 mm and 17 mm, respectively, and an MCF value of 19 mm implying a good hemostatic reserve and pointing toward a thrombocytopenic picture.
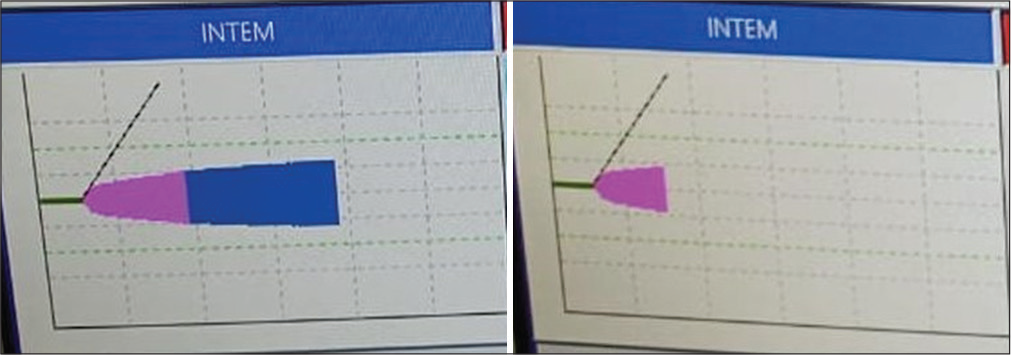
- In INTEM and HEPTEM CT prolonged both pointing toward platelet dysfunction.
DISCUSSION
In study of our center in 2021–2022 on pediatric cardiac surgical patients, we observed that no episode of clot formation in the CPB circuit, thromboembolism, or anaphylactic reaction was observed. Excessive bleeding occurred in four children, one of whom required re-exploration. A neonate with transposition of great arteries (TGA) underwent re-exploration within 2 h of shifting to the ICU. Continuous bleeding was noticed from the aortic suture lines, which was surgically controlled. Another neonate with TGA, also having aortic arch hypoplasia, underwent an arch reconstruction under deep hypothermic circulatory arrest at 18°C, followed by an arterial switch surgery on hypothermic CPB. After weaning from CPB, continuous bleeding occurred from aortic suture lines and the patient succumbed after 6 h due to acute left ventricular failure. The prolongation of CT in EXTEM and ITEM signifies prolonged bleeding with bivalrudin but no anaphylaxes or clot formation was observed while on bivalirudin.[5] DTI group is bivalirudin, with a rather short half-life of 25 min. It undergoes dual elimination, through proteolytic degeneration and partial renal excretion, completely independent of the liver. Reported initial doses in adult patients range from 0.03 mg/kg/h to 0.5 mg/kg/h, with an average of 0.27 ± 0.37 mg/kg/h, adjusted according to the anticoagulation monitoring. Ranucci et al. found bivalrudin to be safe in post-cardiotomy ECMO patients. Moreover, it may have a better coagulation profile, with fewer hemorrhagic events and transfusions of blood products with a comparable thromboembolic complications rate.[6] A recent systematic review and meta-analysis based on 10 articles and 847 patients investigated the efficacy and safety of bivalrudin compared to UFH, revealing that bivalrudin may significantly reduce the incidence of major bleeding (in children) and thrombotic events, in-circuit thrombosis, and in-hospital mortality. The authors concluded that bivalrudin can be a safe and feasible alternative to UFH, especially in the case of HIT and heparin resistance.[7] Anticoagulation monitoring is usually accomplished using aPTT, ACT, plasma drug concentration, anti-IIa assays, or viscoelastic methods.[8] The main disadvantages of these relatively new drugs include direct drug costs, the lack of a specific reversal agent or antidote, and the potential of destabilization of already existing clots. However, due to the short half-life time, the major disadvantages may have less importance.[9] The use of viscoelastic hemostatic assays is recommended for the guidance of coagulation factors and blood product substitution in patients with hemorrhagic diathesis, usually once a day in the case of ECMO patients.[10,11] Recent observations showed that the hypercoagulable state, as assessed by viscoelastic methods, may predict the risk of thrombotic adverse events. Although the evidence on the use of TEG® and ROTEM® in anticoagulation monitoring and ECMO patient management is increasing, it still exhibits differing results regarding the prediction of thrombosis and bleeding.[10] A recent prospective and observational study reported a moderate correlation of INTEM CT (ROTEM®) with traditional tests, which is superior to TEG®. However, this study was limited by a small sample size (25 patients) and comparison to only aPTT and ACT, as standard coagulation monitoring.[11] Therefore, the future clinical studies comparing viscoelastic methods with traditional monitoring are warranted.
CONCLUSION
Monitoring anticoagulation during bivalirudin therapy is sometimes confounding and challenging. Although bivalirudin prolongs ACT, aPTT, and thrombin time, the underlying hemostatic abnormality cannot be accurately detected using these assays as prolongation of PT, aPTT may be a combination of any underlying issue (including bivalirudin therapy). Another method is measurement of bivalirudin concentration which may not be clinically available; therefore, aPTT, thrombin time, and Ecarin chromogenic assay can be used to monitor therapy. Prolongation of CT on INTEM and HEPTEM has shown moderate to strong correlation with traditional laboratory tests like aPTT and HPTT (aPTT with Hepzyme).[12] ROTEM can be a way forward for monitoring bivalirudin therapy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Anticoagulation with direct thrombin inhibitors during extracorporeal membrane oxygenation. World J Crit Care Med. 2019;8:87-98.
- [CrossRef] [PubMed] [Google Scholar]
- The role of ROTEM variables based on clot elasticity and platelet component in predicting bleeding risk in thrombocytopenic critically ill neonates. Eur J Haematol. 2021;106:175-83.
- [CrossRef] [PubMed] [Google Scholar]
- TEG® and ROTEM® traces: Clinical applications of viscoelastic coagulation monitoring in neonatal intensive care unit. Diagnostics (Basel). 2021;11:1642.
- [CrossRef] [PubMed] [Google Scholar]
- The role of evidence-based algorithms for rotational thromboelastometry-guided bleeding management. Korean J Anesthesiol. 2019;72:297-322.
- [CrossRef] [PubMed] [Google Scholar]
- Bivalirudin anticoagulation in neonates and infants undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2022;36:3841-6.
- [CrossRef] [PubMed] [Google Scholar]
- Bivalirudin-based versus conventional heparin anticoagulation for postcardiotomy extracorporeal membrane oxygenation. Crit Care. 2011;15:R275.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy and safety of bivalirudin versus heparin in the anticoagulation therapy of extracorporeal membrane oxygenation: A systematic review and meta-analysis. Front Pharmacol. 2022;13:771563.
- [CrossRef] [PubMed] [Google Scholar]
- Monitoring of argatroban and lepirudin anticoagulation in critically ill patients by conventional laboratory parameters and rotational thromboelastometry-a prospectively controlled randomized double-blind clinical trial. BMC Anesthesiol. 2018;18:18.
- [CrossRef] [PubMed] [Google Scholar]
- Anticoagulation strategies during extracorporeal membrane oxygenation: A narrative review. J Clin Med. 2022;11:5147.
- [CrossRef] [PubMed] [Google Scholar]
- 2021 ELSO adult and pediatric anticoagulation guidelines. ASAIO J. 2022;68:303-10.
- [CrossRef] [PubMed] [Google Scholar]
- Thromboelastometry, thromboelastography, and conventional tests to assess anticoagulation during extracorporeal support: A prospective observational study. ASAIO J. 2021;67:196-200.
- [CrossRef] [PubMed] [Google Scholar]
- Monitoring bivalirudin therapy in children on extracorporeal circulatory support devices: Thromboelastometry versus routine coagulation testing. Thromb Res. 2020;186:54-7.
- [CrossRef] [PubMed] [Google Scholar]






