Translate this page into:
Recent Improvements in Transfusion Practices in Cardiac Surgery
*Corresponding author: Klaus Görlinger, Department of Anaesthesiology and Intensive Care Medicine, University Hospital Essen, Essen, Germany. kgoerlinger@werfen.com
-
Received: ,
Accepted: ,
How to cite this article: Görlinger K, Petricevic M. Recent Improvements in Transfusion Practices in Cardiac Surgery. J Card Crit Care TSS. 2025;9:4-8. doi: 10.25259/JCCC_58_2024
The development and implementation of evidence-based rotational thromboelastometry (ROTEM)/thromboelastography (TEG)-guided bleeding management algorithms play an essential role in the second pillar of patient blood management (PBM) with the aim to minimize bleeding and blood loss and to improve patients’ outcomes.[1] Accordingly, Kvisselgaard et al. assessed in their systematic review and meta-analysis the efficacy of TEG or ROTEM-guided algorithms in bleeding patients.[2] This systematic review included 31 randomized controlled trials (RCTs; n = 2756), with most patients undergoing elective cardiac surgery. TEG-/ROTEM-guided bleeding management algorithms reduced in the random model; the proportion of patients transfused with packed red blood cells (RBCs) (risk ratio [RR] [95% confidence interval (CI)], 0.93 [0.86–1.01], I2 = 92%), fresh frozen plasma (FFP) (0.5 [0.32–0.72], I2 = 94%), platelets (0.7 [0.55–0.91], I2 = 57%), the incidence of surgical reintervention (0.65 [0.47–0.94], I2 = 0%), the incidence of dialysis-dependent acute renal failure (0.66 [0.33–1.34], I2 = 44%), the amount of bleeding (standard mean difference −0.31 [−0.55–−0.08], P < 0.00001, I2 = 75%), and mortality (0.76 [0.57–1.00], P = 0.05, I2 = 5%). Another meta-analysis on the effect of viscoelastic testing (VET) on mortality in cardiovascular surgery, lung transplant, and extracorporeal membrane oxygenation (ECMO) presented recently at the annual meeting of the American Society of Anesthesiology 2024 in Philadelphia based on 10 RCTs with 876 participants (overall mortality, 7.1%) demonstrated a significant reduction in mortality with a RR (95% CI) of 0.5280 (0.3183– 0.8756) and a P = 0.0204.[3] Accordingly, recent guidelines support the implementation of perioperative treatment algorithms based on viscoelastic point-of-care (POC) testing for the bleeding patient undergoing cardiac [Figure 1a and b] and non-cardiac surgery to reduce the number of transfusions and to improve patients’ outcomes with a level 1A recommendation.[4,5]
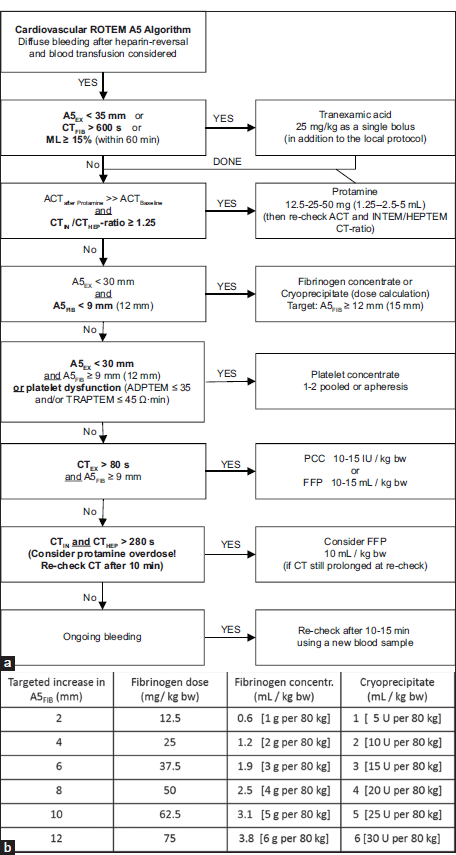
- (a) Evidence-based ROTEM A5 Bleeding Management Algorithm for Adult Patients undergoing Cardiovascular Surgery (Courtesy of Klaus Görlinger, Essen, Germany). A5FIB values in brackets are triggered or targets for patients with severe bleeding, major aortic surgery or situations where higher fibrinogen values should compensate for low platelet counts. (b) Fibrinogen dose calculation based on the targeted increase in FIBTEM A5 (or A10) (Courtesy of Klaus Görlinger, Essen, Germany). Fibrinogen dose (g) = targeted increase in A5FIB (mm) × body weight (kg)/160. The correction factor (140–160 mm kg/g) depends on the actual plasma volume of the patient. Rule of thumb: 1 g Fibrinogen can at best increase A5FIB by 2 mm in an adult patient (with 70–80 kg body weight). 10 U Cryoprecipitate contains about 2 g fibrinogen. 1 L FFP contains about 2–2.5 g fibrinogen. Therefore, FFP is not suitable to treat hypofibrinogenemia. In severe bleeding or delayed therapy (by using cryoprecipitate), the observed A5FIB increase can be (1–3 mm) lower than the calculated increase. A5: Amplitude of clot firmness 5 min after CT, CT: Clotting time, ML: Maximum lysis, ACT: Activated clot time, PCC: Prothrombin complex concentrate, FFP: Fresh frozen plasma, EX = EXTEM, FIB = FIBTEM, IN = INTEM, HEP = HEPTEM, ADPTEM: ROTEM platelet (impedance aggregometry) assay with adenosine di-phosphate-based platelet activation, TRAPTEM: ROTEM platelet (impedance aggregometry) assay with thrombin receptor-activating peptide-based platelet activation, ROTEM: Rotational thromboelastometry.
In this context, the “BloodClot Consensus Recommendations on Bleeding Management during Cardiac Surgery” published by Kapoor et al. in this issue of the Journal of Cardiac Critical Care is an important document dealing with the implementation of bleeding management during cardiac surgery in low-resource settings such as in India.[6] It is important to develop national and local PBM implementation recommendations, particularly in low- to mid-income countries with limited resources such as India and Nepal.[7,8] By implementing and adhering to these PBM recommendations, healthcare professionals can contribute to improved patient safety and outcomes in adult and pediatric cardiovascular surgery.
Besides the development and implementation of practical and reasonable evidence-based bleeding management algorithms, education and knowledge in hemostasis, as well as the diagnostic performance of the VET assays used in these algorithms are crucial [Table 1].[9-12] Here, fast and reliable discrimination between fibrin- and platelet contribution to clot firmness is essential. Research shows that both α-angle and maximum amplitude parameters reflect the combined contribution of fibrinogen and platelets to clot strength.[13] Therefore, although TEG mono-analysis is still used in many institutions and useful for identifying a coagulopathic state, it cannot be used to discriminate between fibrin/fibrinogen and/or platelet deficits, respectively. Conversely, the use of viscoelastic methods where 2 assays can be run simultaneously, one with platelet inhibitors (ROTEM Fibrin-based thromboelastometry test (FIBTEM), ClotPro FIBtest, TEG functional fibrinogen [TEG-FF], or Quantra fibrin contribution to clot stiffness [FCS]) and one without (ROTEM Extrinsically activated thromboelastometric test (EXTEM), ClotPro FIBtest, Rapid-TEG, or quantra clot stiffness) can effectively allow for the identification of specific coagulopathic states, such as insufficient fibrin formation or an insufficient contribution of platelets to clot strength.[10] Such information is critical for making the appropriate decision whether to transfuse fibrinogen concentrate or cryoprecipitate on the one hand or platelets on the other hand. Here, VET assays such as FIBTEM or FIBtest with complete platelet neutralization using the combination of cytochalasin D and tirofiban eliminate “Platelet Noise” in the assay and are superior to the use of a single inhibition of platelets with the GPIIbIIIa receptor inhibitor abciximab (TEG-FF and Quantra FCS).[14] “Platelet Noise” in viscoelastic “functional fibrinogen assays” can result in overestimation of the fibrin contribution to clot firmness and underestimation of the platelet contribution to clot firmness and thereby to inappropriate platelet transfusion when fibrinogen substitution (fibrinogen concentrate or cryoprecipitate) is needed.[11,14]
| VET device | ROTEM delta | ROTEM sigma | TEG 5000/TEG 6s | Quantra |
|---|---|---|---|---|
| Fibrinolysis | HF, FS, and PMCR (EXTEM, FIBTEM, and APTEM ) | HF, FS, and PMCR (EXTEM, FIBTEM, and APTEM) | HF and FS (KaolinTEG, RapidTEG, and TEGFF) | HF (only in QStatCartridge) |
| UFH | ++ | ++ | ++ | ++ (CTR) |
| LMWH | NATEM/NAHEPEM CTratio (>0.1 antiXa units/mL) | INTEM/HEPTEM CTratio (>0.5 antiXa units/mL) | + (KaolinTEG+HeparinaseTEG) | ? |
| Heparin neutralization | EXTEM, FIBTEM, APTEM (Polybrene), and HEPTEM | EXTEM, FIBTEM, APTEM (Polybrene), and HEPTEM | HeparinaseTEG; New Heparin Neutralization Cartridge (FDA, 2024) | CS, FCS, PCS (Polybrene), CTH (Kaolin with Heparinase) |
| Fibrinogen | ++ (Cytochalasin D in FIBTEM) | +++ (Cytochalasin D+Tirofiban in FIBTEM C) | + (Abciximab in TEGFF) | + (Abciximab in FCS) |
| Platelets | ++ (PLTEM=EXTEM–FIBTEM) | +++ (PLTEM=EXTEM–FIBTEM) | + (RapidTEG–TEGFF) | ++ (PCS=CS–FCS) |
| Platelet Function | ROTEM platelet, Multiplate, Platelet Works, VerifyNow | ROTEM platelet, Multiplate, Platelet Works, VerifyNow | TEG Platelet Mapping | Multiplate, Platelet Works, VerifyNow |
| Cirrhosis | ++ | ++ | + | - |
| VKAs | ++ (EXTEM CT) | ++ (EXTEM CT) | (Rapid TEG=Kaolin+Tissue Factor) | (No TFactivated CT available) |
| DOACs | + (EXTEM CT) | + (EXTEM CT) | + (RapidTEG?) | (No TFactivated CT available) |
HF: Hyperfibrinolysis, FS: Fibrinolysis shutdown, PMCR: Plateletmediated clot retraction, TEGFF: Thromboelastography functional fibrinogen, CTR: Clotting time ratio, CS: Clot stiffness, FCS: Fibrin contribution to clot stiffness, PCS: Platelet contribution to clot stiffness, FIBTEM C: FIBTEM in ROTEM sigma Cartridge, VET: Viscoelastic testing, ROTEM: Rotational thromboelastometry, TEG: Thromboelastography, UFH: Unfractionated heparin, LMWH: Lowmolecularweight heparin, DOACs: Direct oral anticoagulants, VKAs: Vitamin KAntagonists, EXTEM: Extrinsically activated thromboelastometric test, FIBTEM: Fibrin-based thromboelastometry test, APTEM: Aprotinin based thromboelastometry test, NATEM: Non-Activated rotational thromboelastometric assay, NAHEPEM: Non-Activated heparinase elastometric assay, INTEM: Intrinsic thromboelastometry test, HEPTEM: Heparinase assay based thromboelastometry test, FDA: Food and drug administration, CTH: Heparinase clot time, +++: very good diagnostic performance, ++: good diagnostic performance, +: acceptable diagnostic perforemance, -:insufficient diagnostic performance, ?: unclear diagnostic performance, CT: Clotting time, TF- activated: Tissue factor-activated, PLTEM: Platelet-specific ROTEM
In patients in which the effect of vitamin K-antagonists, direct oral anticoagulants, or prothrombin complex concentrate (PCC) should be monitored, it is essential that the VET assay fulfills the requirements as characterized by the European Association of Cardiothoracic Anaesthesiology and Intensive Care: The VET assay must be tissue factor-activated, factor VII-dependent and heparin insensitive.[12]
In this context, it is also important to know that protamine overdose can result in significantly prolonged activated clot and clotting time (CT) results in VET, particularly in intrinsically activated assays such as intrinsic thromboelastometry Test (INTEM), heparinase assay based thromboelastometry test (HEPTEM), Kaolin-TEG, Heparinase-TEG, as well as Quantra CT and heparinase clot time (CTH). Overdosing of protamine may contribute to bleeding and increased transfusion requirements by inhibiting factor V – the cofactor of factor X – and platelet function.[15] The available evidence suggests that protamine dosing should not exceed a protamineto-heparin ratio of 1:1. In particular, protamine-to-heparin dosing ratios >1 are associated with more post-operative 12 h blood loss, blood product (RBC, FFP, and platelet) transfusion, and thoracotomy.[16] Accordingly, the optimal ratio between protamine and the initial heparin in cardiac surgery may vary between 0.6 and 1.0. Here, the INTEM/HEPTEM CT ratio correlates well with the heparin concentration (r = 0.72) and can differentiate between activated clot time (ACT) prolongations due to a residual heparin effect or a protamine overdose.[17] This is a low-hanging fruit to avoid post-cardiopulmonary bypass (CPB) bleeding and unnecessary transfusion due to protamine overdose.
Furthermore, in this issue of the Journal of Cardiac Critical Care, Kammerer and Goerlinger published “Ten Recommendations for Managing Bleeding in Paediatric Cyanotic Cardiac Surgery.”[18] They address important issues in bleeding management in this vulnerable patient population, which presents a unique challenge. Particularly, neonates and infants have specific considerations based on age, weight, underlying cardiac disease, physiology, and pharmacology, requiring specific bleeding management and PBM strategies.[19,20]
In summary, the question of whether VETs should be standard POC tests in all intensive care units (ICUs) taking care of bleeding patients – whether adults or children - can be answered with yes. This definitely includes operation theaters and ICUs in low- to mid-income countries since resources of blood products, factor concentrates, and hemostatic drugs are very limited and precious in these countries and, therefore, should be used in the best way possible. This issue of the Journal of Cardiac Critical Care contributes significantly to the education of clinicians in evidence-based bleeding management and to improved patients’ outcomes – not only in low-resource countries.
Conflicts of interest
KG works as the Medical Director of TEM Innovations/Werfen PBM, Munich, Germany.
References
- The Role of Evidence-based Algorithms for Rotational Thromboelastometry-guided Bleeding Management. Korean J Anesthesiol. 2019;72:297-322.
- [CrossRef] [PubMed] [Google Scholar]
- Thromboelastography or Rotational Thromboelastometry Guided Algorithms in Bleeding Patients: An Updated Systematic Review with Meta-analysis and Trial Sequential Analysis. Acta Anaesthesiol Scand. 2025;69:e14558.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Viscoelastic Testing on Mortality in Cardiovascular Surgery, Lung Transplant, and ECMO: A Meta-Analysis. 2024. The Anesthesiology Annual Meeting. Available from: https://www.abstractsonline.com/pp8/#!/20301/presentation/31677 [Last accessed on 2024 Dec 20]
- [Google Scholar]
- EACTS/EACTAIC/EBCP Scientific Document Group 2024 EACTS/EACTAIC Guidelines on Patient Blood Management in Adult Cardiac Surgery in Collaboration with EBCP. Eur J Cardiothorac Surg. 2024;2024:ezae352.
- [CrossRef] [PubMed] [Google Scholar]
- Extract from the 2022 ESC Guidelines on Cardiovascular Assessment and Management of Patients Undergoing Non-cardiac Surgery-Patient Blood Management. Blood Transfus. 2024;22:122-9.
- [Google Scholar]
- Blood Clot Consensus Recommendations on Bleeding Management during Cardiac Surgery in Low-Resource Settings using E-Delphi Methodology. J Card Crit Care TSS. 2025;9:9-20.
- [Google Scholar]
- Patient Blood Management in India-Review of Current Practices and Feasibility of Applying Appropriate Standard of Care Guidelines. A Position Paper by an Interdisciplinary Expert Group. J Anaesthesiol Clin Pharmacol. 2021;37:3-13.
- [CrossRef] [PubMed] [Google Scholar]
- Patient blood Management for Cardiovascular Surgery: Clinical Practice Consensus Statement. Nepal Heart J. 2023;20:33-41.
- [CrossRef] [Google Scholar]
- Transfusion Medicine Knowledge among Clinicians at a Teaching Hospital in Malaysia. Malays J Pathol. 2023;45:187-94.
- [Google Scholar]
- Can the Viscoelastic Parameter. ?-Angle Distinguish Fibrinogen from Platelet Deficiency and Guide Fibrinogen Supplementation? Anesth Analg. 2015;121:289-301.
- [CrossRef] [PubMed] [Google Scholar]
- Trials and Tribulations of Viscoelastic-Based Determination of Fibrinogen Concentration. Anesth Analg. 2020;130:644-53.
- [CrossRef] [PubMed] [Google Scholar]
- A European Consensus Statement on the Use of Four-factor Prothrombin Complex Concentrate for Cardiac and Non-cardiac Surgical Patients. Anaesthesia. 2021;76:381-92.
- [CrossRef] [PubMed] [Google Scholar]
- Admission Rapid Thrombelastography Can Replace Conventional Coagulation Tests in the Emergency Department: Experience with 1974 Consecutive Trauma Patients. Ann Surg. 2012;256:476-86.
- [CrossRef] [PubMed] [Google Scholar]
- FIBTEM PLUS Provides an Improved Thromboelastometry Test for Measurement of Fibrin-based Clot Quality in Cardiac Surgery Patients. Anesth Analg. 2013;117:1054-62.
- [CrossRef] [PubMed] [Google Scholar]
- Anticoagulant and Side-effects of Protamine in Cardiac Surgery: A Narrative Review. Br J Anaesth. 2018;120:914-27.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of High or Low Protamine Dosing on Postoperative Bleeding Following Heparin Anticoagulation in Cardiac Surgery. A Randomised Clinical Trial. Thromb Haemost. 2016;116:251-61.
- [CrossRef] [PubMed] [Google Scholar]
- Reappearance of Circulating Heparin in Whole Blood Heparin Concentration-based Management Does not Correlate with Postoperative Bleeding After Cardiac Surgery. J Cardiothorac Vasc Anesth. 2014;28:1003-7.
- [CrossRef] [PubMed] [Google Scholar]
- Ten Recommendations for Managing Bleeding in Paediatric Cyanotic Cardiac Surgery. J Card Crit Care
- [CrossRef] [Google Scholar]
- Perioperative Paediatric Patient Blood Management: A Narrative Review. Br J Anaesth. 2025;134:168-79.
- [CrossRef] [PubMed] [Google Scholar]
- Perioperative Considerations in the Paediatric Patient with Congenital and Acquired Coagulopathy. BJA Open. 2024;12:100310.
- [CrossRef] [PubMed] [Google Scholar]





