Translate this page into:
Post-operative External Compression of the Right Coronary Artery Following Triple Valve Surgery
*Corresponding author: Douglas Tran, Department of Cardiac Surgery, University of Maryland Medical Center, Baltimore, United States. douglas.tran@som.umaryland.edu
-
Received: ,
Accepted: ,
How to cite this article: Tran D, Ho K, Shah A, Zapata D. Post-operative External Compression of the Right Coronary Artery Following Triple Valve Surgery. J Card Crit Care TSS. 2025;9:130-3. doi: 10.25259/JCCC_9_2025
Abstract
We describe a 66-year-old male with right ventricular dysfunction, elevated troponins, and ST segment changes on electrocardiogram following a triple valve repair. Coronary angiography revealed a discrete right coronary artery stenosis that was refractory to multiple angioplasty attempts. Intravascular ultrasound showed no signs of intraluminal plaque or thrombosis. Recognizing the possibility of an external compressive process, the patient’s chest tubes were removed, successfully resolving the stenosis. This case emphasizes the importance of thoughtful placement of any temporary material around the heart and the need for a high index of suspicion for external compression on native coronary anatomy.
Keywords
Aortic valve
Mitral valve
Right coronary artery
Tricuspid valve
INTRODUCTION
Valve replacement and repair procedures frequently lead to complex post-operative challenges. Among these, right ventricular (RV) dysfunction and myocardial infarction (MI) are particularly morbid due to their significant impact on patient recovery. Studies show that RV dysfunction occurs in approximately 19.7% of patients undergoing left-sided valve surgeries, while MI affects about 26% of patients undergoing aortic valve replacements.[1,2]
The diagnosis of RV dysfunction involves a comprehensive assessment, including echocardiography, hemodynamic monitoring, and clinical signs. MI following cardiac surgery involves a nuanced assessment that goes beyond standard diagnostic criteria. The pattern of troponin release after cardiac procedures varies widely and is influenced by several intraoperative factors. The duration of cross-clamp time, the extent of pre-existing coronary artery disease, and the type of cardioplegia strategy employed all influence myocardial injury and the resultant troponin elevation.
Distinguishing between a normal post-operative myocardial injury and a true post-operative MI requires a careful evaluation of troponin trends in conjunction with other clinical and electrocardiogram (ECG) findings, factoring in the specific surgical context. Echocardiography plays a major role in delineating the diagnosis as it can effectively show any changes in RV function or new wall motion abnormalities that would prompt further investigation through a diagnostic left heart catheterization (LHC).
We present a unique case where the placement and trajectory of chest tubes following triple valve surgery played a role in the development of acute RV dysfunction, highlighting an underrecognized and rare cause of this condition. This retrospective case study was conducted in accordance with ethical standards and approved by the Institutional Review Board at the University of Maryland.
CASE REPORT
A 66-year-old male with a history of ST-elevation myocardial infarction post-percutaneous coronary intervention, non-ST-elevation myocardial infarction, pulmonary hypertension, and chronic kidney disease (Stage IIIb), presented with dyspnea, orthopnea, and volume overload. A transesophageal echocardiogram (TEE) revealed an ejection fraction (EF) of 55%, severe mitral regurgitation, moderate tricuspid regurgitation, moderate aortic regurgitation, and severe pulmonary hypertension. Pre-operative LHC showed minimal luminal irregularities, a patent circumflex, and a mid-left anterior descending stent with no obstructive disease. The right coronary artery was dominant with a mild 10–20% disease.
The patient underwent mitral valve and aortic valve replacement with a tricuspid valve repair and closure of a patent foramen ovale. Intraoperative TEE showed an EF of 40–45% and lateral-inferior wall hypokinesis after initial weaning from cardiopulmonary bypass, which improved with inotropic support and pacing before chest closure. There were no significant valvular gradients, paravalvular leaks, or ECG changes at this time or after chest closure.
On post-operative day 2, the patient exhibited signs of worsening RV dysfunction, including decreased urine output, decreased mixed venous saturations, and increased central venous pressures. Transthoracic echocardiogram showed moderately reduced RV function and severely reduced left ventricular EF of 25–30% with mid to apical segmental inferior akinesis consistent with right coronary artery disease. Rising troponins from 48.5 ng/mL on postoperative day 1–103 ng/mL, and ECG changes showing inferior Q waves and 1 mm ST elevations [Figure 1], led to a LHC for suspected MI.
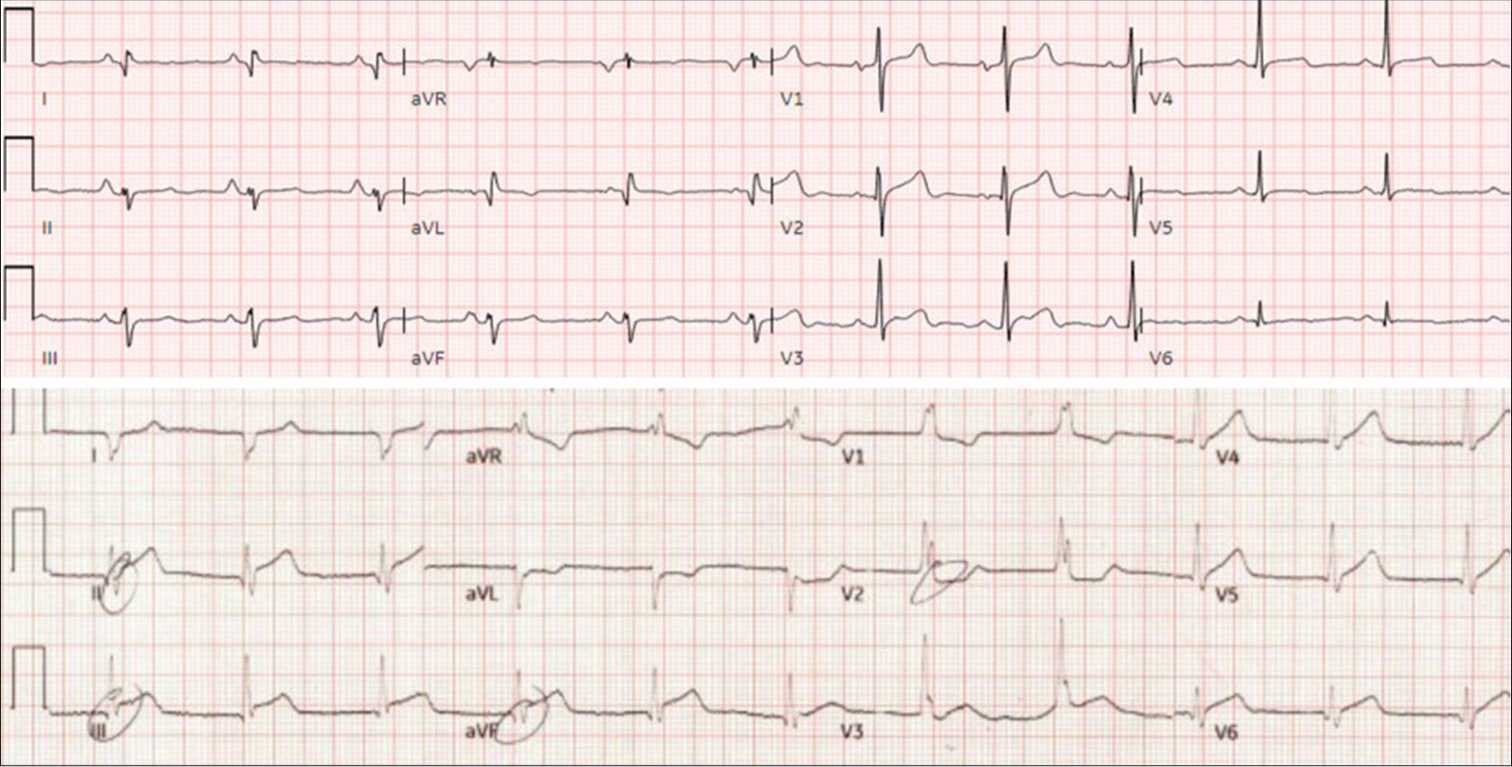
- Pre- and post-operative electrocardiogram (EKG), Top: Pre-operative EKG, Bottom: postoperative EKG with new ST-elevations in leads II, III, and aVF.
LHC revealed a discrete new mid-posterior descending artery lesion with 99% stenosis. Initial attempts to dilate the lesion with a 2.5 mm semi-compliant balloon showed minimal improvement. A 3.0 × 20 mm semi-compliant balloon also failed to improve the stenosis despite good angiographic expansion [Figure 2].
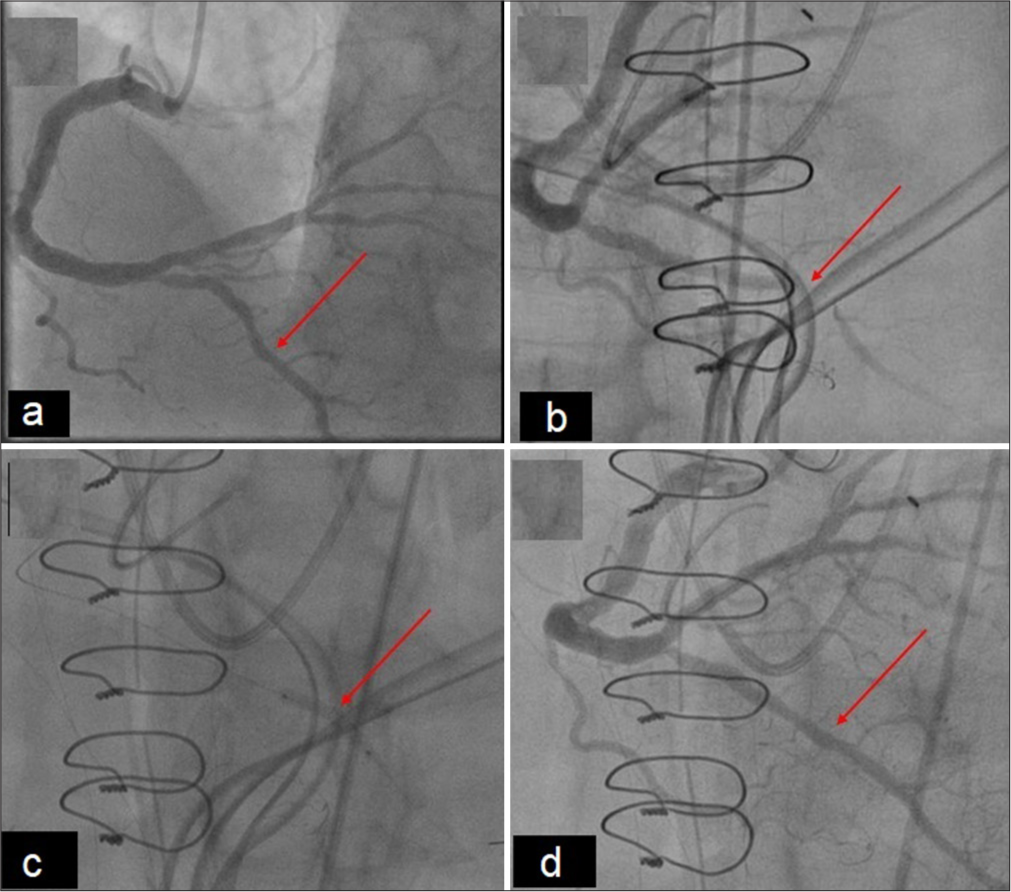
- Pre- and post-operative left heart catheterization: Right coronary artery anatomy. (a) Pre-operative images of the left heart catheterization (LHC) showing no significant right sided coronary obstruction of the posterior descending artery (PDA) (red arrow) and (b) Post-operative LHC with the focus on the right coronary artery. The red arrow indicates the placement of the Blake drain over the PDA following angioplasty with delayed filling still present. (c) Post-operative LHC with the focus on the right coronary artery. The red arrow indicated the attempted balloon angioplasty that did not resolve the obstruction. (d) Post-operative LHC following Blake drain removal. Notable resolution of stenosis and delayed filling of the PDA (red arrow).
Intravascular ultrasound (IVUS) showed no significant luminal plaque or thrombosis over the stenosed segment, suggesting an external compressive process [Figure 3]. This was confirmed after the sequential removal of the patient’s two pericardial Blake drains, resulting in complete resolution of the lesion. The remainder of the hospital course was uneventful, and the patient was discharged home on post-operative day 23.
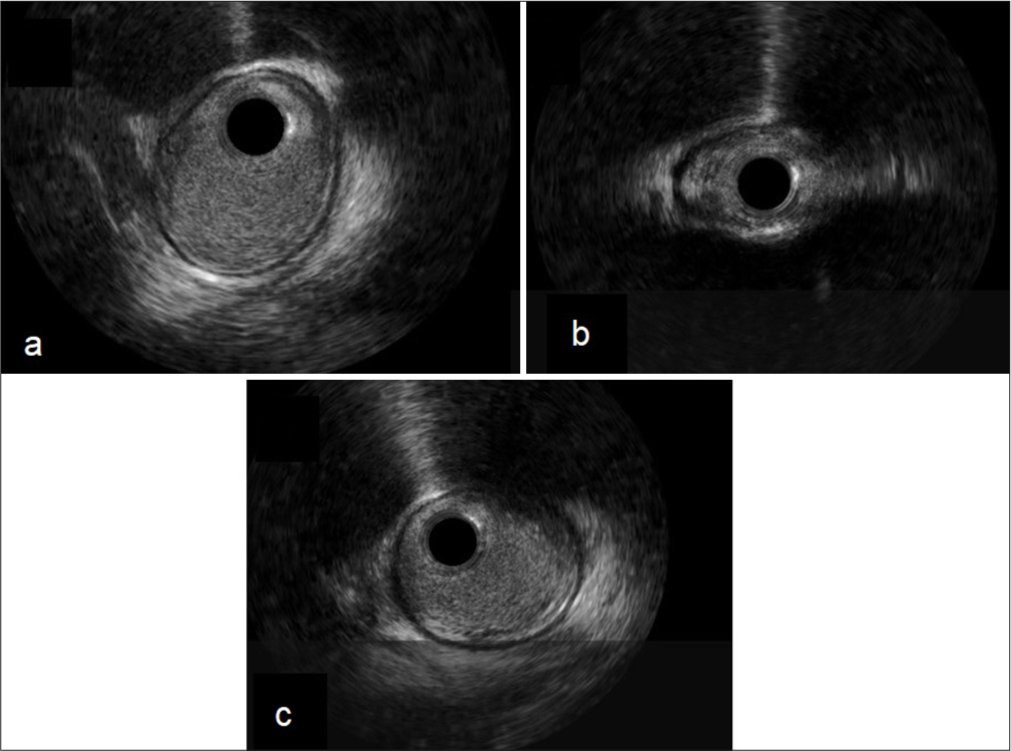
- Right coronary intravascular ultrasound (IVUS). (a) Pre-lesion IVUS: Proximal posterior descending artery, (b) Lesion IVUS: Mid posterior descending artery, and (c) Post-lesion: Distal posterior descending artery.
DISCUSSION
The patient’s symptoms-including rising troponins, RV dysfunction, and ST elevations suggested a post-operative inferior MI. Common causes of post-operative MI include acute destabilization and rupture of pre-existing atherosclerotic plaque, coronary embolization, acute coronary thrombosis, or direct injury to the coronary artery during surgery.[3] The incidence rate is 2–10% due to the high-risk nature of cardiac procedures.[4]
RV dysfunction is typically caused by alterations in preload, afterload, and contractility.[3] Its etiology includes volume and pressure overload, embolic or inflammatory events, oxidative stress, and ischemic injury from prolonged cardiopulmonary bypass and cross-clamping.[5] Although intraoperative RV dysfunction did occur, it improved before chest closure. Subsequent RV dysfunction was delayed, presenting over 24 h after chest closure, indicating a low likelihood of direct coronary injury. Several features in this patient presentation suggested an alternative diagnosis.
The sudden stenosis post-surgery and discrete narrowing of the right coronary artery on angiography were atypical for atherosclerotic lesions. The patient’s pre-operative assessment showed no significant right coronary artery disease, making acute plaque rupture unlikely. Atherosclerotic plaques develop gradually and have a diffuse, irregular appearance on imaging, unlike the localized, smooth narrowing seen in this case. The lesion’s resistance to multiple angioplasty attempts and the absence of luminal plaque confirmed by IVUS ruled out atherosclerosis or luminal thrombus, supporting the diagnosis of external compression. The stenosis‘s resolution following the removal of two pericardial Blake drains confirmed external compression as the cause.
Meticulous attention to cardiac anatomy is crucial when placing chest tubes or packing around the heart postoperatively. Excessive pressure or compression can lead to myocardial ischemia, arrhythmias, or cardiac dysfunction. Frequent reassessment of chest tube positioning ensures they do not exert excessive force on the heart or coronary anatomy, particularly on vulnerable coronary segments with superficial courses.
CONCLUSION
This case emphasizes the importance of considering external compression in post-operative patients with acute myocardial ischemic signs. A high index of suspicion, a low threshold for coronary angiography and IVUS, and timely identification and intervention are essential to significantly reduce the risk of severe morbidity and mortality from this rare complication.
Ethical approval
The research/study approved by the Institutional Review Board at University of Maryland Medical Center, number HP-00076929, dated 6th October, 2024.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Right Ventricular Dysfunction and Short-Term Outcomes Following Left-sided Valvular Surgery: An Echocardiographic Study. J Am Heart Assoc. 2021;10:e016283.
- [CrossRef] [PubMed] [Google Scholar]
- Post-procedural Myocardial Infarction Following Surgical Aortic Valve Replacement and Transcatheter Aortic Valve Implantation. EuroIntervention. 2017;13:e153-60.
- [CrossRef] [PubMed] [Google Scholar]
- European Association of Cardio-Thoracic Surgery (EACTS) Expert Consensus Statement on Perioperative Myocardial Infarction after Cardiac Surgery. Eur J Cardiothorac Surg. 2024;65:ezad415.
- [CrossRef] [PubMed] [Google Scholar]
- Myocardial Infarction after Cardiac Surgery: When to Intervene? J Thorac Cardiovasc Surg. 2023;165:1195-201.
- [CrossRef] [PubMed] [Google Scholar]
- Perioperative Right Ventricular Dysfunction and Abnormalities of the Tricuspid Valve Apparatus in Patients Undergoing Cardiac Surgery. J Clin Med. 2023;12:7152.
- [CrossRef] [PubMed] [Google Scholar]







