Translate this page into:
POCUS and Fluid Responsiveness on Venoarterial ECMO
*Corresponding author: Sanchita Garg, Senior Resident, Department of Cardiac Anaesthesiology and Critical Care, Cardiac Thoracic Centre, All India Institute of Medical Sciences, New Delhi, India. doc.sanchita@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Garg S, Kapoor P. POCUS and fluid responsiveness on venoarterial ECMO. J Card Crit Care TSS 2023;7:40-7.
Abstract
VA ECMO allows organ perfusion and oxygenation while awaiting myocardial recovery, cardiac transplantation, or long-term mechanical circulatory support. Diagnosis of hospital-acquired pneumonia (HAP) is a daily challenge for the clinician managing patients on venoarterial ECMO. Lung ultrasound (US) can be a valuable tool as the initial imaging modality for the diagnosis of pneumonia. Point-of-care US (POCUS) is broadly used in patients with ARDS. POCUS is recommended to be performed regularly in COVID-19 patients for respiratory failure management. In this review, we summarized the US characteristics of COVID-19 patients, mainly focusing on lung US and echocardiography. Point-of-care lung US (LUS) was demonstrated to be an effective tool in case of acute respiratory failure for ICU patients, community-acquired pneumonia, and ventilator-associated pneumonia. This review describes the usefulness of LUS in the early detection of HAP in cardiac critically ill patients under VA ECMO as well as assess its sonographic features.
Keywords
POCUS
Fluid responsiveness
VA ECMO
Ultrasound guided
INTRODUCTION
Venoarterial extracorporeal membrane oxygenation (VA ECMO) is an effective rescue therapy providing temporary cardiac and respiratory support for patients with refractory cardiogenic shock. VA ECMO allows organ perfusion and oxygenation while awaiting myocardial recovery, cardiac transplantation, or long-term mechanical circulatory support. Diagnosis of hospital-acquired pneumonia (HAP) is a daily challenge for the clinician managing patients on venoarterial ECMO. Lung ultrasound (US) can be a valuable tool as the initial imaging modality for the diagnosis of pneumonia. Color Doppler intrapulmonary flow and dynamic air Broncho gram appear to be particularly insightful for the diagnosis of HAP.[1] COVID-19 has inflicted the world for over 2 years. The recent mutant virus strains pose greater challenges to disease prevention and treatment. COVID-19 can cause acute respiratory distress syndrome (ARDS) and extrapulmonary injury. Dynamic monitoring of each patient’s condition is necessary to timely tailor treatments, improve prognosis, and reduce mortality.
Point-of-care US (POCUS) is broadly used in patients with ARDS. POCUS is recommended to be performed regularly in COVID-19 patients for respiratory failure management. In this review, we summarized the US characteristics of COVID-19 patients, mainly focusing on lung US and echocardiography. Furthermore, we also provided the experience of using POCUS to manage COVID-19-related ARDS.[2] Significant progress in device technology and in the management of critically ill patients have allowed an international expansion of the use of VA ECMO.[3] However, short-term mortality remains high, with an overall survival rate of 40%, mainly due to the very high incidence of complications, among which infections are predominant.[4] Early diagnosis of HAP in VA ECMO patients remains a daily challenge.
Point-of-care lung US (LUS) was demonstrated to be an effective tool in case of acute respiratory failure for ICU patients,[5,6] community-acquired pneumonia,[7,8] and ventilator-associated pneumonia.[9,10] The LUS diagnosis of ventilator-associated pneumonia in intensive care units is more challenging in comparison with the diagnosis of community-acquired pneumonia in emergency departments due to the limited access to the mechanically ventilated patients and the high prevalence of atelectasis. This review describes the usefulness of LUS in the early detection of HAP in cardiac critically ill patients under VA ECMO as well as assess its sonographic features.
In a busy, resource-constrained intensive care unit (ICU) or emergency room, POCUS can allow triage of patients such that unnecessary costly and time-consuming investigations and interventions can be avoided. By allowing the intensivists to perform these assessments at the bedside, POCUS reduces the risks associated with transportation of critical patients. It also mitigates the risks inherent with exposure to ionizing radiation. An additional advantage is that these assessments can be repeated periodically and can thus be used to assess the patient’s dynamic response to interventions and therapies and can lead to improved outcomes.[11-14]
IMPORTANCE OF POCUS AND FLUID RESPONSIVENESS (FR) ON ECMO
Shock is a potentially life-threatening condition and if not treated promptly, it can lead the patient into a rapid downward spiral ending in death.[15] Intravenous fluids (IVF) are considered the first-line therapy for shock and are routinely used in ICUs and hospitals to restore effective blood volume and maintain organ perfusion.
Patients are transfused fluids with the premise that increasing stressed venous volume and consequently improving stroke volume and cardiac output will result in better tissue oxygenation and organ function. In the early 70s, when the use of the pulmonary artery catheter was in vogue and fluid therapy was being titrated to fixed targets of the central venous pressure (CVP) or pulmonary artery occlusion pressure (PAOP).[16] The landmark EGDT (early goal directed therapy) study by Rivers et al.[17] showed that massive fluid administration (30 mL/kg) during the first 6°h of resuscitation of patients with severe sepsis and septic shock improved outcomes. This study heralded an era of large volume resuscitation and rigid targeted protocols.
However, there is a growing body of evidence[18-23] that has shown that indiscriminate use of fluids without giving due regard to the patient’s hemodynamic status and response to the purported boluses can be just as detrimental to the patient’s health. The harmful effects of a positive fluid balance can present in the form of pulmonary edema, hypoxemia, tissue edema, renal dysfunction, intraabdominal hypertension, delirium, cerebral edema, intestinal dysfunction, impaired wound healing, prolonged ventilator days, and hospital stay, and has been shown to increase mortality as well.[20-23] Recent literature has in fact shown that maintaining a negative fluid balance can improve the chances of survival in patients with septic shock and acute kidney injury.[24] It is important; therefore, to determine whether giving more fluids will result in benefit or harm.
When tissue perfusion is threatened, it is prudent to ascertain which of the three appropriate choice is: Optimization of preload status (fluids), ionotropic support and vasopressors, modulation of afterload, or a combination of the above.
Studies have shown that only about half the hemodynamically unstable patients in a critical care unit will be fluid responsive.[25] To make matters worse, the FENICE investigators[26] found that majority of the clinicians used fluids in an empirical, liberal, and unstructured manner, without due consideration to response to fluid challenges. The time has come that IVFs are given the same respect as that afforded to any other pharmaceutical preparation and should be given only after due assessment.
A patient whose stroke volume or cardiac output (CO) rises by a fixed percentage (commonly 10–15%), in response to a predetermined volume of fluid challenge (commonly bolus 500 mL, 100 mL in mini-fluid challenge), over a predetermined period of time is defined to be “fluid responsive.” Several validated tools and technologies exist today that allow assessment and continuous monitoring of FR, such as those based analysis on arterial pulse contour,[27] transpulmonary thermosdilution, and bioreactance. Although quite a few show promise, most of these need invasive lines and expensive monitors that carry with them their own inherent risks such as pneumothorax and central line associated blood stream infections. The need of the hour is a tool that is inexpensive, non-invasive, easily accessible, and fairly accurate, with reproducible results. The European Society of Intensive Care Medicine has in fact issued a consensus statement on circulatory shock, where in it was proposed that bedside echocardiography be used as a first-line modality in the evaluation of patients with shock.
Theoretically speaking, it appears simple to give fluids to patients that lie on the steep part of the Frank Starling curve and to restrict fluids for patients on the flat part of the curve. However, it is not always possible to pinpoint the patient’s position on the curve, especially when the steepness of the curves varies with changes in the left ventricular (LV) systolic function. A static parameter/marker is measured at a given LV function and presumed to reflect preload at a given point on the Frank-Starling curve. It also assumes that a lower value of preload implies FR. Evidence supports discontinuing the use of static markers of preload, such as CVP and PAOP, because one isolated value does not predict FR.[28] Dynamic indices, on the other hand, involve the delivery of a preload challenge and therefore assess the actual response of the cardiovascular system to the said challenge.[29] This preload challenge could be external (fluid bolus), internal provoked (end expiratory occlusion or passive leg raising [PLR]), or provoked spontaneously by mechanical ventilation.
In addition, there is a physiological variability in the dynamic parameters secondary to variations in intrathoracic pressure occurring during both spontaneous and mechanical breaths. Positive pressure ventilation by increasing the intrathoracic pressure during inspiration, decreases the right ventricular (RV) preload and consequently decreases the RV stroke volume (as described by the Frank-Starling relationship). These phenomena are transmitted to the LV pressures after pulmonary transit time. This manifests during expiration as a decrease in LV preload and LV stroke volume.[30] These changes in stroke volume caused due to heart-lung interactions are monitored before and after a preload challenge. The change is more pronounced when the patient is preload dependent; greater the volume deficit, the larger is the change in the dynamic parameters. The magnitude of the changes indicates the patient’s position the Frank-Starling curve. Heart-lung interactions therefore form the basis of most tests for FR. This forms the basis of the multitude of stroke volume variation (SVV) monitoring systems and can also be assessed by calculating the flow through valves, vessels, or outflow tracts using Doppler echocardiography. There are certain prerequisites to be fulfilled for SVV and its derivatives to be good predictors of FR. These limitations hold true for all continuous cardiac output monitors as well. Few echocardiographic indices can overcome these limitations.
There are several POCUS-guided indices that help the clinician ascertain the state of FR. Some of these are explained below and others are described [Tables 1-5 and Figures 1-4].

- Venoarterial extracorporeal membrane oxygenation circuit.
![(a) Diagram showing probe positions for focused abdominal ultrasound (US). (Probe positions; (1): Subcostal, (2): Right upper quadrant, (3): left upper quadrant, (4) Pelvic; (b) Blue Points. (1) Just below clavicle (upper BLUE point). (2) Close to the nipple (lower BLUE point). (3) Junction of the horizontal line from the lower BLUE point and the posterior axillary line (posterolateral alveolar and/or pleural syndrome [PLAPS] point).](/content/149/2023/7/1/img/JCCC-7-040-g002.png)
- (a) Diagram showing probe positions for focused abdominal ultrasound (US). (Probe positions; (1): Subcostal, (2): Right upper quadrant, (3): left upper quadrant, (4) Pelvic; (b) Blue Points. (1) Just below clavicle (upper BLUE point). (2) Close to the nipple (lower BLUE point). (3) Junction of the horizontal line from the lower BLUE point and the posterior axillary line (posterolateral alveolar and/or pleural syndrome [PLAPS] point).
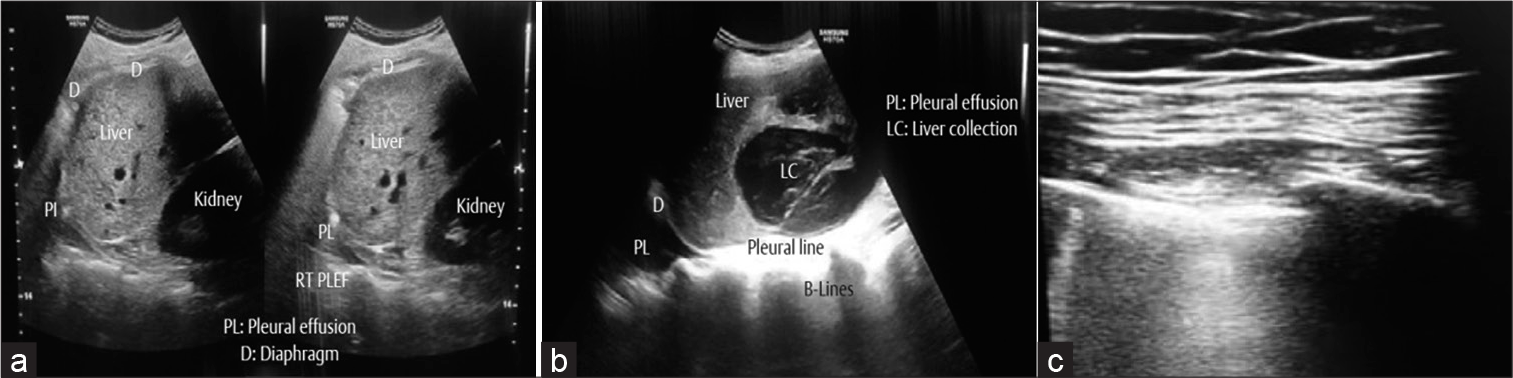
- (a) Point-of-care ultrasound (POCUS) showing the relations of right lung, liver, and right kidney. A small right-sided pleural effusion. (b) POCUS showing large hepatic collection and a right-sided pleural effusion. (c) Confluent multiple B-lines suggesting pneumothorax on extracorporeal membrane oxygenation.

- Abdominal Ultrasonography showing liver with perihepatic fluid.
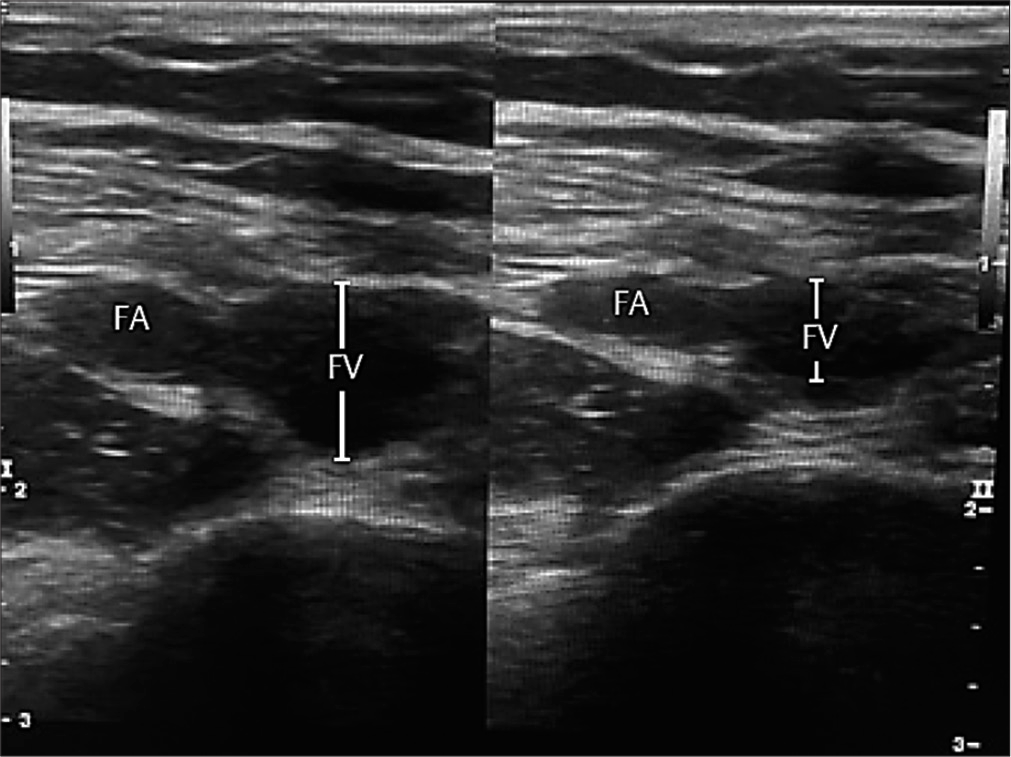
- Compressible femoral vein indicates absence of deep venous thrombosis.
| Name of Protocol | Purpose/Utility | Views involved | Abnormalities detected |
|---|---|---|---|
| BLUE: Bedside lung ultrasound in emergency[9] | Diagnosis in acute respiratory failure | LUS | A-profile B-profile |
| FALLS: Fluid administration limited by lung sonography protocol[10] | Management of unexplained shock | CUS BLUE protocol |
Sequentially rules out obstructive, cardiogenic, hypovolemic shock, and finally as exclusion distributive shock |
| Sonography in hypotension and cardiac arrest protocols[11] | Two protocols, one for hypotension and the other for cardiac arrest | CUS LUS IVC |
pericardial fluid, cardiac form and ventricular function AAA or DVT |
| C.A.U.S.E: Cardiac arrest ultra-sound exam[12] | Rule out causes of cardiac arrest (non-arrhythmogenic) |
LUS CUS |
Pericardial tamponade Tension pneumothorax Pulmonary embolus Hypovolemia |
| SESAME protocol[13] | Sequential echographic scanning assessing mechanism or origin of severe shock of indistinct cause | CUS LUS IVC AUS DVT |
|
| GUCCI: Global ultrasound check for the critically III[14] | diagnose and differentiate between the most common ICU syndromes (acute respiratory failure, shock, and cardiac arrest) | CUS LUS IVC AUS DVT |
Rules out common diagnoses and incorporates US-guided procedures such as thoracocentesis and pericardiocentesis |
| PIEPEAR workflow[15] | 7-step protocol with decision and management tree for cardiorespiratory compromise | LUS, AUS/DVT, CUS | Includes a complex 7 step algorithm that deals with pathophysiology, etiology and actions needed. |
| ACES: Abdominal and cardiac evaluation with sonography in Shock | Establish diagnosis and deliver goal-directed therapy for Non-traumatic undifferentiated shock in ED | Cardiac, peritoneal, pleural, inferior vena cava and aortic views | Common causes of shock |
| VExUS: Venous excess ultrasound grading system of the severity of venous congestion | Evaluates venous congestion in the IVC, liver, kidneys, gut and correlates with risk of AKI | IVC Hepatic, portal and renal vein Dopplers | 0–3 VExUS grades |
POCUS: Point-of-care ultrasound, LUS: Lung ultrasound, CUS: Cardiac ultrasound, 4 views 4C: 4 chamber view, IVC: Inferior vena cava, DVT: Deep vein thrombosis, AAA: Abdominal aortic aneurysm
| For spontaneously breathing patients IVC COLLAPSIBILITY INDEX=(maximum diameter on expiration – minimum diameter on inspiration)/maximum diameter on expiration |
| For mechanically ventilated patients IVC DISTENSIBILITY INDEX=(maximum diameter on inspiration–minimum diameter on expiration)/minimum diameter on expiration |
IVC: Inferior vena cava
| IVC diameter | IVC variation | Estimated CVP | Recommendation for fluids |
|---|---|---|---|
| >2.5 cm | <50% collapse | 15–20mm Hg | Not recommended |
| 1.5–2.5 cm | <50% collapse | 10–20mm Hg | Indeterminate |
| 1.5–2.5 cm | >50% collapse | 10–20mm Hg | Indeterminate |
| ≤2.5 cm | >50% collapse | 0–5 mm Hg | Should be given |
IVC: Inferior vena cava, CVP: Central venous pressure
| Index | View | Interpretation |
|---|---|---|
| LVEDA | PSAX | ≤10 cm2: significant hypovolemia ≥20 cm2:volume overload |
| E/A ratio | 4C A | E/A >2, DT<160s: PCWP >18 mmHg |
| IVC Dia | SC | ≤10 mm: CVP <5–10 mm Hg ≥20 mm: CVP >15–20 mm Hg |
POCUS: Point-of-care ultrasound, LVEDA: Left ventricular end-diastolic area, LVEDI: Left ventricular end-diastolic index, IVC Dia: IVC diameter, PSAX: Parasternal short axis view, 4C A: Apical 4 chamber view, SC: Subcostal view, CVP: Central venous pressure
| Index | View | Threshold value (∆) | Advantages | Limitations |
|---|---|---|---|---|
| ∆LV area | PSAX | >16% | Easy to perform | Image acquisition |
| IVC D | SC | Easy to perform | RV dysfunction: tamponade, RV infarct Obesity Open chest cavity ↑IAP Big swings in ITP Image acquisition |
|
| IVC C | SC | >18% | ||
| ∆VmaxAo | 5C A | >12% | Spontaneously breathing patients TV <8 mL/kg High RR (HR/RR<3.6) Poor lung compliance, ARDS Arrhythmias Open chest cavity ↑ IAP |
|
| VTI | ≥20% | |||
| PLR | 5C A | >10±2% | Can be used in: Spontaneously breathing patients with arrhythmias | ↑IAP ↑ ICP Pregnancy Open chest cavity Lower limb/pelvic fractures |
| ∆SVC | longitudinal 90−100−view | >36% | Can be used in: Spontaneously breathing patients with arrhythmias patients with ↑IAP | Availability and access Training Invasive Upper airway or esophageal disease Image acquisition Skill set |
| EEO | >5% | Easy to perform Can be used in ARDS |
Patients not intubated |
POCUS: Point-of-care ultrasound, ∆: Delta or change, ∆LV area: Left ventricular area, IVC D: IVC distensibility IVC C: IVC collapsibility, VTI: Velocity time integral, ∆Vmax Ao: Variation in peak aortic flow velocity, EEO: End-expiratory occlusion test, ∆SVC: Variations of the diameter of the SVC PLR: Passive leg raising, ∆IVC: Variations of the diameter of the inferior vena cava, PLAX: Parasternal long axis view, 5C A: Apical 5 chamber view, SC: Subcostal view PSAX: Parasternal short axis view, Threshold value: which differentiates between fluid responders and non-responders. HR: Heart rate, ↑ IAP: Increased intra-abdominal pressure, ↑ ICP: Increased intra-cranial pressure, RR: Respiratory rate
LV size (LV end diastolic area and index)
The ventricular size, best judged visually (eyeballing) in parasternal short axis view, can give a rough estimate of patient’s preload state. Fluid response is to be expected in small, chinked or kissing ventricles (papillary muscles seem to meet each other at end-systole) and is unlikely when dilated poorly contracting ventricles are observed. LV end diastolic area can also be measured but cutoff values are yet to be suggested.[31]
Inferior vena cava assessment
In a spontaneously breathing patient, inspiration causes the IVC to collapse and vice versa during exhalation. The reverse is true in a mechanically ventilated patient.[32] IVC diameter and its respiratory variation have been extensively studied and can be used to estimate CVP semi-quantitatively [Table 5 and Figure b]. Use of respiratory variations in IVC diameter to predict FR has been validated in both mechanically ventilated (distensibility index) and spontaneously breathing patients (collapsibility index). However, as with CVP measurements, recommendations are still unclear due to several confounding factors. It is most useful when the values at the extremes. The formula is (Dmax–Dmin/Dmin) × 100. Current literature casts doubts about validity of respiratory variation of IVC as an accurate index for FR.[33]
Superior vena cava (SVC) assessment (using transesophageal echocardiography [TEE])
Respiratory variability in the SVC diameter can be assessed using TEE. The main disadvantage of this approach is that its use is restricted to sedated and mechanically ventilated patients because of assessment using TEE. It has the potential advantage to avoid all confounding elements associated with changes in intra-abdominal pressure, concerns regarding spontaneous respiratory efforts, and can even be used in patients with irregular cardiac rhythms. As compared to assessment respiratory variability in IVC, the SVC (cutoff >36%) performs better as a marker for FR in terms of sensitivity and specificity.[34] The distensibility index used for respiratory variation in mechanically ventilated patients and its formula is (Dmax–Dmin/Dmin) × 100.
Aortic blood flow variations
Stroke volume can be estimated by multiplying area of LV outflow tract (LVOT) with velocity-time integral (VTI), using a pulsed wave (PW) Doppler signal. The LVOT area can be measured from the parasternal long axis view and PW Doppler signal acquired in the apical five-chamber view.[35] The LVOT area is assumed to be constant; therefore, changes in VTI, averaged over three respiratory cycles, can be used to as a surrogate of SVV. This index has been validated with as little as 100 mL of hydroxyethyl starch as a bolus given over 1 min.
With the assumption that LVOT area is constant, changes in aortic blood flow would be proportional to changes in stroke volume. Peak aortic blood flow velocity can be estimated using continuous wave or PW Doppler with the sweep speed set to include several respiratory cycles in a screen.
PLR test
Another innovative test designed to detect FR is the PLR test.[36] The PLR examines the impact of an internal preload challenge to estimate changes in stroke volume and determine FR. The main advantages are that it can be employed reliably even in spontaneously breathing patients, patients with irregular rhythms, and has been used in patients receiving ECMO. An added benefit is that no external volume is added to the circulation. There are some technical challenges faced in maintaining correct probe angle with LVOT. It is not reliable in the presence of raised intra-abdominal pressure, which precludes use in many surgical patients.[37] It is also not feasible in patients who are pregnant, have lower limb fractures, and is not recommended in patients with raised intracranial pressure.
End-expiratory occlusion test
The changes in preload caused during regular respiration are normally transmitted from the right-sided to left-sided circulation after one pulmonary transit time. By stopping mechanical ventilation for more 15 s, there is a transient increase in cardiac preload. If the end-expiratory occlusion test results in an increase in CO or SV, it indicates FR.[38]
Corrected carotid flow time index
Corrected carotid flow time index is calculated as a ratio of the systolic flow time and square root of the cardiac cycle time (to correct for impact of heart rate). A pulsed-wave Doppler waveform of carotid blood flow is generated and the flow time between the onset of systole and dicrotic notch. A fluid bolus or PLR associated with an increase in the CFTI value 10–15% indicates fluid FR.[39]
Is fluid to be given always on VA ECMO?
In some instances, such as an actively bleeding polytrauma patient or early untreated septic shock, FR is obvious. Don’t waste time. FR is not to be tested when CO needs to be increased for reasons other than circulatory shock, example, for a case of tissue hypoxia. All patients who are deemed FR cannot be given fluids. Sometimes, the benefits of fluid administration are greatly outweighed by the risks. For instance, in a patient with ARDS and circulatory shock, or ischemic or dilated cardiomyopathy with poor LV function and septic shock. In such situations, repeated assessments with added emphasis on assessment of extravascular lung water are needed. Don’t drown the patient. No test is 100% sensitive or specific. Always correlate clinically. Treat the patient not the test result. Our study demonstrates that lung US is a useful tool as an initial imaging modality for the diagnosis of pneumonia in patients on VA ECMO and is probably more powerful than chest radiography. LUS is rapid and easy to perform at the bedside, in addition to being non-invasive and relatively inexpensive. As shown in [Figure 5], a deep vein thrombus on VA ECMO can be ruled out by details POCUS too.
CONCLUSION
Over the past decade, there has been an increasing emphasis on patient safety and evidence-based medicine. Protocolized patient care has been shown to decrease errors, standardize patient care, and improve outcomes. In recent years, the scope and usage of US have expanded to the extent that POCUS has been considered by some as the modern stethoscope. If used judiciously, US-based protocols that incorporate screening of multiple organ systems can impact the accuracy of the patient’s diagnosis and also hasten the management of critically ill patients.
Declaration of patient consent
Patient’s consent not required as their identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Usefulness of lung ultrasound for early detection of hospital-acquired pneumonia in cardiac critically ill patients on venoarterial extracorporeal membrane oxygenation. Ann Intensive Care. 2022;12:43.
- [CrossRef] [PubMed] [Google Scholar]
- Application of POCUS in patients with COVID-19 for acute respiratory distress syndrome management: A narrative review. BMC Pulm Med. 2022;22:52.
- [CrossRef] [PubMed] [Google Scholar]
- Extracorporeal membrane oxygenation: Evolving epidemiology and mortality. Intensive Care Med. 2016;42:889-96.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality in patients with cardiogenic shock supported with VA ECMO: A systematic review and meta-analysis evaluating the impact of etiology on 29,289 patients. J Heart Lung Transplant. 2021;40:260-8.
- [CrossRef] [PubMed] [Google Scholar]
- Lung ultrasound in critically ill patients: Comparison with bed side chest radiography. Intensive Care Med. 2011;37:1488-93.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9-15.
- [CrossRef] [PubMed] [Google Scholar]
- Lung ultrasound in community-acquired pneumonia and in interstitial lung diseases. Respir Int Rev Thorac Dis. 2014;87:179-89.
- [CrossRef] [PubMed] [Google Scholar]
- Lung ultrasound in diagnosing pneumonia in the emergency department: A systematic review and meta-analysis. Eur J Emerg Med. 2018;25:312-21.
- [CrossRef] [PubMed] [Google Scholar]
- Specifc ultrasound signs may improve bedside early diagnosis of ventilator-associated pneumonia. Respir Care. 2019;64:1175-6.
- [CrossRef] [PubMed] [Google Scholar]
- Lung ultrasound for diagnosis and monitoring of ventilator-associated pneumonia. Ann Transl Med. 2018;6:418.
- [CrossRef] [PubMed] [Google Scholar]
- Should the ultrasound probe replace your stethoscope? A SICS-I sub-study comparing lung ultrasound and pulmonary auscultation in the critically ill. Crit Care. 2020;24:14.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation and adaptation of clinical practice guidelines. Evid Based Nurs. 2005;8:68-72.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized controlled clinical trial of point-of-care, limited ultrasonography for trauma in the emergency department: The first sonography outcomes assessment program trial. Ann Emerg Med. 2006;48:227-5.
- [CrossRef] [PubMed] [Google Scholar]
- Limited echocardiography-guided therapy in subacute shock is associated with change in management and improved outcomes. J Crit Care. 2014;29:700-5.
- [CrossRef] [PubMed] [Google Scholar]
- Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165-228.
- [CrossRef] [PubMed] [Google Scholar]
- Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172-8.
- [CrossRef] [PubMed] [Google Scholar]
- Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-77.
- [CrossRef] [PubMed] [Google Scholar]
- Review of a large clinical series: Association of cumulative fluid balance on outcome in acute lung injury: A retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med. 2009;24:35-46.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of different crystalloid volume regimes on intestinal anastomotic stability. Ann Surg. 2009;249:181-5.
- [CrossRef] [PubMed] [Google Scholar]
- Secondary abdominal compartment syndrome: A potential threat for all trauma clinicians. Injury. 2007;38:272-9.
- [CrossRef] [PubMed] [Google Scholar]
- Fluid management in critically ill patients: The role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann Intensive Care. 2012;2(Suppl 1 Diagnosis and management of intra-abdominal hyperten):S1.
- [CrossRef] [PubMed] [Google Scholar]
- Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259-65.
- [CrossRef] [PubMed] [Google Scholar]
- Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care. 2013;17:R246.
- [CrossRef] [PubMed] [Google Scholar]
- Negative fluid balance predicts survival in patients with septic shock: A retrospective pilot study. Chest. 2000;117:1749-54.
- [CrossRef] [PubMed] [Google Scholar]
- Predicting fluid responsiveness in ICU patients: A critical analysis of the evidence. Chest. 2002;121:2000-8.
- [CrossRef] [PubMed] [Google Scholar]
- Fluid challenges in intensive care: The FENICE study: A global inception cohort study. Intensive Care Med. 2015;41:1529-37.
- [CrossRef] [Google Scholar]
- Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: A systematic review of the literature. Crit Care Med. 2009;37:2642-7.
- [CrossRef] [PubMed] [Google Scholar]
- Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41:1774-81.
- [CrossRef] [PubMed] [Google Scholar]
- Perioperative cardiovascular monitoring of high-risk patients: A consensus of 12. Crit Care. 2015;19:224.
- [CrossRef] [PubMed] [Google Scholar]
- Heart-lung interactions during mechanical ventilation: The basics. Ann Transl Med. 2018;6:349.
- [CrossRef] [PubMed] [Google Scholar]
- Echocardiography to guide fluid therapy in critically ill patients: Check the heart and take a quick look at the lungs. J Thorac Dis. 2017;9:477-81.
- [CrossRef] [PubMed] [Google Scholar]
- The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30:1834-7.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy of ultrasonographic measurements of inferior Vena Cava to determine fluid responsiveness: A systematic review and meta-analysis. J Intensive Care Med. 2020;35:354-63.
- [CrossRef] [PubMed] [Google Scholar]
- Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med. 2004;30:1734-9.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119:867-73.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: Systematic review and meta-analysis of clinical studies. Intensive Care Med. 2010;36:1475-83.
- [CrossRef] [PubMed] [Google Scholar]
- Predicting fluid responsiveness by passive leg raising: A systematic review and meta-analysis of 23 clinical trials. Crit Care Med. 2016;44:981-91.
- [CrossRef] [PubMed] [Google Scholar]
- Functional hemodynamic tests: A systematic review and a metanalysis on the reliability of the endexpiratory occlusion test and of the mini-fluid challenge in predicting fluid responsiveness. Crit Care. 2019;23:264.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound assessment of the change in carotid corrected flow time in fluid responsiveness in undifferentiated shock. Crit Care Med. 2018;46:e1040-6.
- [CrossRef] [PubMed] [Google Scholar]






