Translate this page into:
Impact of an Evidence-Based Antibiotic Protocol on Common Gram-Negative Bacteria's Antibiotic Resistance in a Cardiac Surgical Intensive Care Unit
Sarvesh Pal Singh, DM Cardio-Neuro Sciences Center, All India Institute of Medical Sciences, Room No 2 8th Floor, New Delhi India 110029 sarveshpal.singh@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background Based on the analysis of infections and antibiotic usage in the years 2013 and 2014, an evidence-based antibiotic protocol was developed and implemented in our cardiac surgical intensive care unit (CSICU). This study intends to study the impact of this new protocol on the sensitivity profiles of common gram-negative bacteria in our CSICU.
Methods The medical records of patients who underwent cardiac surgery at our center, between January 2017 and December 2018, were reviewed and the incidence of different hospital-acquired bacteria and their antibiotic sensitivity profiles were recorded. The antibiotic-sensitivity profiles of common gram-negative bacteria, for the years 2017 and 2018, were compared with the published data of 2013 and 2014 from our department.
Results There was a significant decrease in the incidence of Acinetobacter baumannii, Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa resistant to carbapenems during 2017 and 2018. The incidence of colistin-resistant A. baumannii and P. aeruginosa also decreased significantly in 2017 and 2018. A significant increase in the proportion of amikacin resistant A. baumannii and E. coli and A. baumannii resistant to B lactam/B lactamase inhibitors also occurred.
Conclusion Antibiotic stewardship can reverse the antibiotic resistance of common gram-negative bacteria in the ICU.
Keywords
antibiotic
stewardship
resistance
intensive
care
Introduction
Antibiotic resistance is increasing day by day. Carbapenem-resistant (CR) Acinetobacter baumannii, CR Pseudomonas aeruginosa, and third-generation cephalosporin and CR Enterobacteriaceae are considered to be an urgent threat for the world. The World Health Organization accorded the highest priority for the research and development of new antibiotics against these bacteria.1, 2 The problem, which was earlier limited to the intensive care units, is now a public health problem.3, 4 The scoping report on India's antimicrobial resistance has emphasized the emergence of colistin resistance in India.5 According to a study by Kaur et al, bloodstream infections caused by organisms resistant to both carbapenems and colistin lead to 69.3% mortality in Indians.6 The ICMR AMR (Indian Council of Medical Research antimicrobial resistance) surveillance data suggests that resistance to fluoroquinolones and third-generation cephalosporin is more than 70% in A. baumannii, Escherichia coli, and Klebsiella pneumoniae, and more than 50% in P. aeruginosa. Similarly, 70% of A. baumannii, 57% of K. pneumoniae, 41% of P. aeruginosa, and 16% of E. coli were resistant to carbapenems.5 A study was done in the Department of Cardiothoracic and Vascular Surgery (CTVS), AIIMS, New Delhi, including patients from the years 2013 and 2014 that reported the incidence of nosocomial infections and sepsis-related mortality as 4.6 and 1.9%, respectively.7 The authors of this study also reported 28 and 18% resistance to colistin in E. coli and Acinetobacter spp., respectively. Gandra et al reported a 3 to 4% resistance to colistin in Klebsiella, E. coli, and Acinetobacter in 2016.
The “Scoping report on antimicrobial resistance in India” published in 2017 focused on the burden of antibiotic resistance in various groups (e.g., neonates) in different healthcare settings and recommended developing and studying the impact of antimicrobial stewardship activity in healthcare facilities.5 We designed and implemented an evidence-based antibiotic protocol in our intensive care unit (ICU) in 2015. This study intends to evaluate this antibiotic protocol's impact on the incidence and resistance patterns of common gram-negative bacteria in our cardiac surgical intensive care unit (CSICU).
Methodology
This study's primary objective was to elucidate the change in the incidence of common gram-negative bacteria's antibiotic resistance in our CSICU after implementing an evidence-based antibiotic protocol.
After obtaining permission from the institute ethics committee, this retrospective study was conducted at our CSICU. The reference number for ethical clearance is IEC 132/06.03.2020; RP-31/2020.
The medical records of patients who were operated in the Department of CTVS between January 2017 and December 2018 (2 years) were reviewed. Patients who developed an infection (of any site) were included in this study. A frequency of more than 10% (out of total samples included in the study) was used as a cutoff to define common gram-negative bacteria. Infection was defined as a significant growth (on culture media) of an organism from bodily fluids or tissue sent for bacterial cultures.
For analysis, the incidence of different hospital-acquired bacteria and their antibiotic sensitivity profiles is recorded. The samples sent for culture were tracheal secretions, bronchoalveolar lavage fluid, blood (central venous and/or arterial), urine, fluid from intercostal drainage tube, peritoneal dialysis catheter, mediastinal tube, and tissue from surgical site. Reports of patients with different pathological bacteria in separate samples were logged in as independent reports for analysis. Reports, where multiple bacteria were identified in one sample, were very few and excluded from the analysis.
A descriptive study by Sahu et al was conducted in 2015 to study the incidence of different hospital-acquired infections and causative bacteria in our CSICU. Based on the analysis of infection and antibiotic usage in the years 2013 and 2014, evidence-based antibiotic protocol (Fig. 1) was designed for the CSICU at our center. This antibiotic protocol was implemented in 2015, but it took almost a year for the protocol to be entirely in place. Since 2016, this protocol is routinely followed in our CSICU.
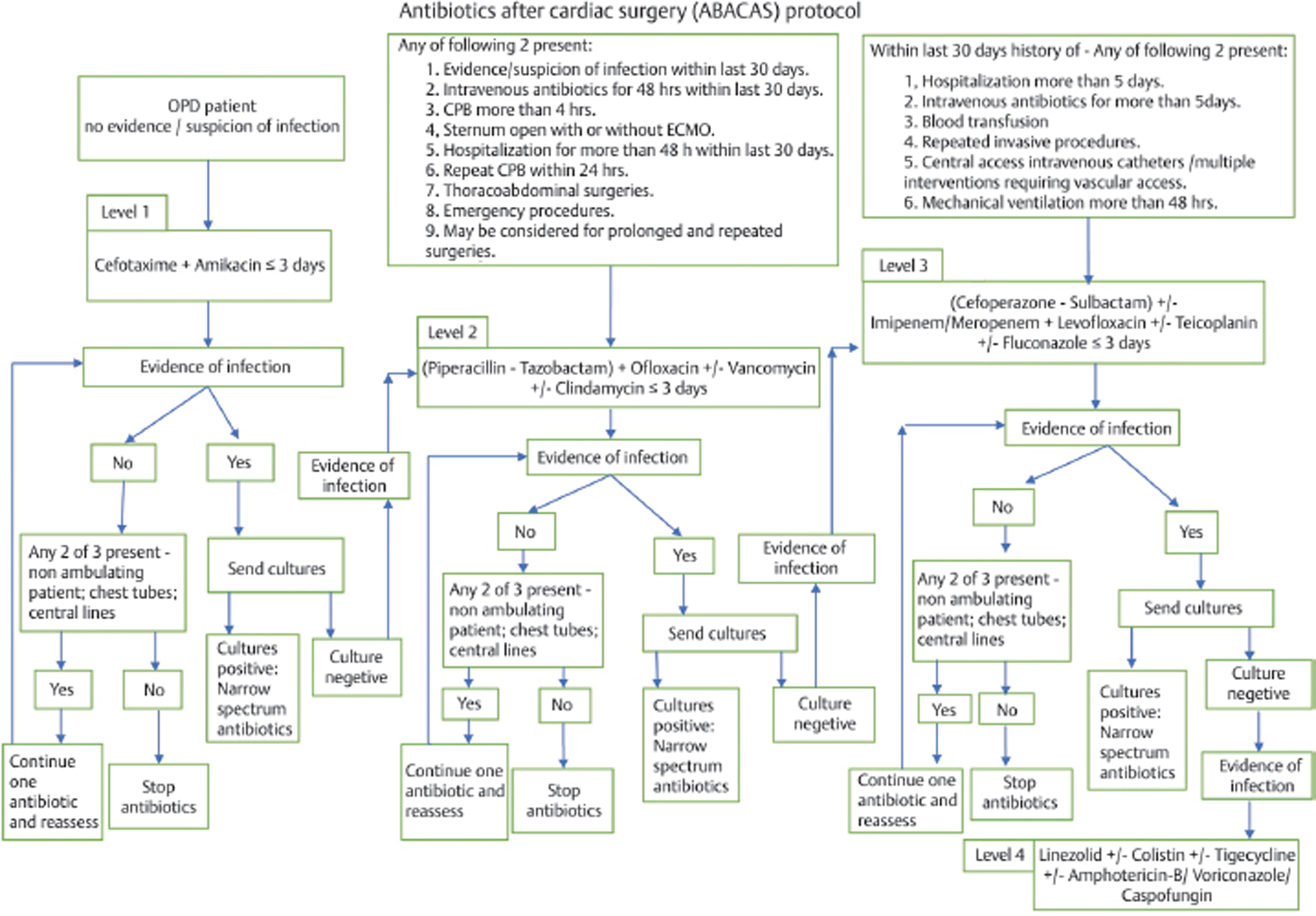
- Antibiotic protocol. CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; OPD, outpatient department.
This study analyzed the effect of this antibiotic protocol on the incidence of different hospital-acquired bacterial infections and the change in these bacteria's resistance patterns to the available antibiotics. To evaluate the effect of this new antibiotic protocol, the incidence of various infective bacteria and their antibiotic-resistant profiles for the year 2017 and 2018 was recorded and compared with the published data of 2013 and 2014 from our department only. However, for comparison, only common gram-negative bacteria whose resistance profile was published earlier by Sahu et al were included in this study.7 In contrast to the study of Sahu et al, which included postoperative patients from ICU only, our study included patients from both ICU and high dependency units and patients who were readmitted to wards or ICUs after discharge?
Chi-squared test was used to compare proportions of resistant and nonresistant bacteria between the years 2013 to 2014 and 2017 to 2018. A p-value of <0.05 was considered significant for intergroup comparisons.
Results
The reports for a total of 1,080 samples were included in the study. Table 1 shows the different bacteria identified in various samples. Gram-negative bacteria with >10% prevalence were statistically analyzed. Acinetobacter baumannii, K. pneumoniae, E. coli, and P. aeruginosa were common bacteria with a percentage of 29.0, 23.2, 18.3, and 11.8% respectively. Other species of Acinetobacter and Klebsiella were not included in the analysis as the number of patients was very less (4–10). Table 2 details the resistance profiles of these common gram-negative bacteria for the years 2013 to 2014 and 2017 to 2018.
|
S. No. |
Bacteria |
n(%) |
|---|---|---|
|
1 |
Acinetobacter baumannii |
314 (29.0) |
|
2 |
Klebsiella pneumoniae |
251 (23.2) |
|
3 |
Escherichia coli |
198 (18.3) |
|
4 |
Pseudomonas aeruginosa |
128 (11.8) |
|
5 |
Staphylococcus spp. (including CONS) |
97 (8.9) |
|
6 |
Enterobacter spp. |
29 (2.6) |
|
7 |
Chryseobacterium spp. |
11 (1.0) |
|
8 |
Stenotrophomonas maltophilia |
16 (1.5) |
|
9 |
Proteus spp. |
6 (0.5) |
|
10 |
Aeromonas hydrophila |
3 (0.3) |
|
11 |
Burkholderia cepacia |
4 (0.3) |
|
12 |
Citrobacter spp. |
1 (0.1) |
|
13 |
Shewanella putrefaciens |
2 (0.2) |
|
14 |
Providencia rettgeri |
5 (0.4) |
|
15 |
Serratia marcescens |
4 (0.3) |
|
16 |
Morganella morganii |
5 (0.4) |
|
17 |
Elizabethkingia meningoseptica |
4 (0.3) |
|
18 |
Alcaligenes faecalis |
1 (0.1) |
|
19 |
Ralstonia pickettii |
1 (0.1) |
|
Total |
1080 |
|
S. No |
Acinetobacter baumannii (%) |
Klebsiella pneumoniae (%) |
Escherichia coli (%) |
Pseudomonas aeruginosa (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
2013–2014 |
2017–2018 |
p-Value |
2013–2014 |
2017–2018 |
p-Value |
2013–2014 |
2017–2018 |
p-Value |
2013–2014 |
2017–2018 |
p-Value |
||
|
1 |
Amikacin |
98/121 (81) |
243/265 (91.7) |
<0.0001 |
54/63 (86) |
179/226 (79.2) |
0.002 |
38/45 (84) |
93/184 (50.5) |
0.008 |
26/43 (60) |
43/114 (37.7) |
<0.0001 |
|
2 |
Amoxicillin + clavulanic acid |
119/121 (98.4) |
36/40 (90) |
0.045 |
15/15 (100) |
176/180 (97.8) |
– |
24/25 (96) |
108/112 (96) |
0.317 |
47/49 (96) |
2/5 (40) |
0.083 |
|
3 |
Cefotaxime |
135/135 (100) |
282/295 (95.6) |
– |
77/77 (100) |
206/225 (91.5) |
– |
33/33 (100) |
182/183 (99.4) |
– |
51/51 (100) |
1/5 (20) |
0.025 |
|
4 |
Ceftazidime |
136/136 (100) |
262/278 (94.2) |
– |
78/78 (100) |
213/225 (94.7) |
– |
53/53 (100) |
181/183 (98.9) |
– |
53/53 (100) |
47/113 (41.6) |
0.014 |
|
5 |
Ciprofloxacin |
125/128 (97.7) |
260/283 (91.8) |
0.083 |
70/76 (92) |
174/226(77) |
0.014 |
48/50(96) |
169/178 (94.9) |
0.157 |
48/52 (92) |
47/108 (43.5) |
0.317 |
|
6 |
Cefoperazone + sulbactam |
70/98 (71.4) |
35/289 (12.1) |
<0.0001 |
53/62 (85) |
138/219 (63) |
0.002 |
22/51(43) |
90/172 (52.3) |
<0.0001 |
33/43 (77) |
37/111 (33.3) |
0.045 |
|
7 |
Imipenem |
118/137 (86.1) |
213/272 (78.3) |
<0.0001 |
68/75 (90) |
61/206 (29.6) |
0.008 |
53/55(96) |
20/174 (11.5) |
<0.0001 |
46/54 (85) |
41/111 (36.9) |
0.025 |
|
8 |
Meropenem |
104/127 (81.9) |
196/286 (68.5) |
<0.0001 |
62/72 (86) |
143/223 (64.1) |
0.001 |
43/51 (84) |
121/180 (67.2) |
0.004 |
37/51 (72) |
46/113 (40.7) |
0.002 |
|
9 |
Netilmicin |
126/134 (94) |
9/11 (81.8) |
0.157 |
69/73 (95) |
123/157 (78.3) |
0.045 |
32/38 (84) |
65/157 (41.4) |
0.014 |
40/47 (85) |
12/24 (50) |
<0.0001 |
|
10 |
Piperacillin + tazobactam |
73/116 (62.9) |
253/283 (89.3) |
<0.0001 |
56/73 (77) |
149/223 (66.8) |
<0.0001 |
15/41 (36) |
135/177 (76.3) |
<0.0001 |
25/48 (52) |
49/121 (40.5) |
<0.0001 |
|
11 |
Colistin |
23/126 (18.3) |
0/129 (0) |
<0.0001 |
3/71 (4) |
0/123 (0) |
0.083 |
1/48 (2) |
0/32 (0) |
0.317 |
9/32 (28) |
2/50 (4) |
0.008 |
|
12 |
Tigecycline |
68/106 (64.2) |
0/15 (0) |
<0.0001 |
3/29 (10) |
1/8 (12.5) |
0.157 |
1/14(7) |
0/9 (0) |
0.317 |
3/12 (25) |
0/1(0) |
0.317 |
Chi-squared test was used to compare the antibiotic resistance between the years 2013 to 2014 and 2017 to 2018 (Table 2). All four gram-negative bacteria were 100% resistant to cefotaxime and ceftazidime, in the reported samples, in 2013 to 2014. The chi-squared test could not calculate p-value for three organisms (A. baumannii, K. pneumoniae, and E. coli) for the above two antibiotics. The resistance of A. baumannii, K. pneumoniae, and E. coli to cefotaxime decreased by 4.4, 8.5 and 0.6%, and for ceftazidime decreased by 5.8, 5.3, and 1.1%, respectively.
Acinetobacter baumannii
In comparison to 2013 to 2014, there was a statistically significant increase of 10.7 and 26.4% in the resistance of Acinetobacter to amikacin (p < 0.001) and piperacillin tazobactam (p < 0.001), respectively, in 2017 to 2018. The decrease in resistance to ciprofloxacin (5.9%, p = 0.08) and netilmicin (12.2%, p = 0.15) was not statistically significant. However, compared with 2013 to 2014, in 2017 to 2018, there was a statistically significant decrease in the resistance to amoxicillin–clavulanic acid, cefoperazone sulbactam, imipenem, meropenem, colistin, and tigecycline (Table 2).
Klebsiella pneumoniae
In 2017 to 2018, compared with 2013 to 2014, the resistance of K. pneumoniae decreased significantly for amikacin, ciprofloxacin, cefoperazone sulbactam, imipenem, meropenem, netilmicin, and piperacillin tazobactam (Table 2). The decrease in resistance for colistin was not statistically significant (p = 0.08). There was no significant increase in resistance for tigecycline (12.5 vs. 10%). No statistical significance could be calculated for amoxicillin-clavulanic acid because of 100% resistance in 2013 to 2014 reports (nonresistant bacteria were zero).
Escherichia coli
The resistance of E. coli, in 2017 to 2018, increased significantly for cefoperazone sulbactam and piperacillin tazobactam. The resistance significantly decreased for amikacin, imipenem, meropenem, and netilmicin. The decrease in resistance of E. coli for antibiotics ciprofloxacin, colistin, and tigecycline was not statistically significant (Table 2). The resistance of E. coli for amoxicillin–clavulanic acid was similar for both 2013 to 2014 and 2017 to 2018 (96%).
Pseudomonas aeruginosa
The decrease in resistance of P. aeruginosa, in 2017 to 2018, for amikacin, cefotaxime, ceftazidime, cefoperazone sulbactam, imipenem, meropenem, netilmicin, piperacillin tazobactam, and colistin was statistically significant (Table 2). There was no significant decrease in the resistance for amoxicillin clavulanic acid, ciprofloxacin, and tigecycline.
Discussion
Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Escherichia coli are the four most common (>10% prevalence) gram-negative bacteria in our ICU. Twelve antibiotics (Table 2) were chosen for statistical analysis because there were adequate samples for these antibiotics to derive any conclusion. In our study, the incidence of antibiotic resistance in these four gram-negative bacteria is similar to reported by Feretzakis et al for all antibiotics (Aminoglycosides, Fluoroquinolones, B lactam/ B lactamase combinations, and carbapenems) except Colistin.8
Regarding resistance, in gram-negative bacteria, the antibiotics that have been at the center of research are Cephalosporins, carbapenems, and colistin. An earlier study published from our institute documented the incidence of various antibiotic-resistant bacteria in our CSICU (Table 2). Following this previous study, an antibiotic protocol was formulated and implemented to optimize antibiotic use and prevent any increase in the antibiotic-resistant bacteria. The current study demonstrated that, after implementing the protocol, there was a significant decrease in the incidence of A. baumannii, K. pneumoniae, E. coli, and P. aeruginosa resistant to carbapenems. There was a significant decrease in the incidence of colistin-resistant A. baumannii and P. aeruginosa (p ≤ 0.001 and ≤0.05, respectively) in 2017 to 2018. The incidence of colistin-resistant K. pneumoniae and E. coli in 2013 to 2014 was 4 and 2%, respectively. Therefore, no significant decrease could be demonstrated in 2017 to 2018 for these two bacteria as the number was very less. However, the reversal of resistance to carbapenems has led to a decreased use of colistin in recent years.
The very use of antibiotics increases the possibility of developing antibiotic resistance. The use of the same combination of antibiotics for prolonged periods and more frequent use of one antibiotic ultimately results in resistance to the entire class.9 Antibiotic cycling does not decrease antibiotic resistance in ICU patients. On the other hand, antibiotic stewardship reduces selection pressure on one class of antibiotics and decreases antibiotic resistance.10, 11 Zhang et al showed a decrease in multidrug-resistant strains of K. pneumoniae (34.3 to 20%) and E. coli (35.2 to 19.0%) with antibiotic stewardship. Similarly, antibiotic stewardship decreased the incidence of carbapenem-resistant strains of K. pneumoniae, P. aeruginosa, and A. baumannii from 94 to 6%, 91 to 83%, and 38 to 2.5%, respectively.8 Feretzakis et al8 also demonstrated a reciprocal increase in the incidence of nonresistant isolates of above GNB, during the period, when resistant strains were decreasing.
Strict surveillance should be followed for the emergence of new resistant pathogens while implementing a new antibiotic protocol.4, 12 In our study, most of the resistant bacterial strains decreased in number, but there was a significant increase in the proportion of amikacin-resistant A. baumannii and E. coli and A. baumannii resistant to B lactam/B lactamase inhibitors. With the implementation of a new protocol, the use of aminoglycosides and B lactam/B lactamase inhibitors (1st- and 2nd-level antibiotics) in our CSICU increased. This increased usage could be responsible for the increase in A. baumannii and E. coli resistant to the above antibiotics. Similar findings for carbapenems have been reported as increased usage leads to the emergence of CR Enterobacteriaceae.13
Newer antibiotics, B lactam/B lactamase inhibitors, and non-B lactam, for treating antibiotic-resistant gram-negative bacteria, are being evaluated for safety and efficacy.14 Therefore, in the current scenario, antibiotic stewardship and infection control practices are the mainstay to prevent morbidity and mortality due to antibiotic-resistant bacteria.14
Conclusion
Antibiotic stewardship can reverse the antibiotic resistance of common gram-negative bacteria in the intensive care unit.
Declarations
Ethics Approval
The study was approved by Institute Ethics committee of All India Institute of Medial Sciences, New Delhi, India (IEC 132/06.03.2020; RP-31/2020).
Conflict of Interest Statement
The authors declare they have no competing interests.
Funding None.
References
- Kadri SS. Key takeaways from the U.S. CDC’s 2019 antibiotic resistance threats report for frontline providers. Crit Care Med 2020;48(7):939–945
- WHO Pathogens Priority List Working Group. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318-327.
- [Google Scholar]
- World Health Organization. Regional office for South-East Asia. Jaipur declaration on antimicrobial resistance; 2011. Available from:http://www.who.int/iris/handle/10665/ 205397, last accessed on February 13, 2020
- Multidrug-resistant gram-negative bacterial infections in the hospital setting: overview, implications for clinical practice, and emerging treatment options. Microb Drug Resist. 2016;22(5):412-431.
- [Google Scholar]
- 2017. Scoping Report on Antimicrobial Resistance in India. Washington, DC: Center for Disease Dynamics, Economics & Policy
- Clinical outcome of dual colistin- and carbapenem-resistant Klebsiella pneumoniae bloodstream infections: a single-center retrospective study of 75 cases in India. Am J Infect Control. 2017;45(11):1289-1291.
- [Google Scholar]
- Incidence, microbiological profile of nosocomial infections, and their antibiotic resistance patterns in a high volume cardiac surgical intensive care unit. Ann Card Anaesth. 2016;19(2):281-287.
- [Google Scholar]
- A 2-year single-centre audit on antibiotic resistance of Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae strains from an intensive care unit and other wards in a general public hospital in Greece. Antibiotics (Basel). 2019;8(2):62.
- [Google Scholar]
- Microbial pattern and antimicrobial resistance, a surgeon’s perspective: retrospective study in surgical wards and seven intensive-care units in two university hospitals in Cairo, Egypt. Dermatology. 2006;212(01):8-14.
- [Google Scholar]
- Impact of an antimicrobial stewardship programme on antibiotic usage and resistance in a tertiary hospital in China. J Clin Pharm Ther. 2017;42(5):579-584.
- [Google Scholar]
- SATURN consortium. The effects of antibiotic cycling and mixing on antibiotic resistance in intensive care units: a cluster-randomised crossover trial. Lancet Infect Dis. 2018;18(4):401-409.
- [Google Scholar]
- Reduction in the incidence of methicillin-resistant Staphylococcus aureus and ceftazidime-resistant Klebsiella pneumoniae following changes in a hospital antibiotic formulary. Clin Infect Dis. 1999;28(5):1062-1066.
- [Google Scholar]
- Correlations of antibiotic use and carbapenem resistance in Enterobacteriaceae. Antimicrob Agents Chemother. 2013;57(10):5131-5133.
- [Google Scholar]
- New drugs for multidrug-resistant gram-negative organisms: time for stewardship. Drugs. 2019;79(7):705-714.
- [Google Scholar]






