Translate this page into:
ICU Readmission in Cardiac Surgical Subset: A Problem Worth Pondering
Rohan Magoon, DM, MD Department of Cardiac Anaesthesia, Atal Bihari Vajpayee Institute of Medical Sciences (ABVIMS) and Dr. Ram Manohar Lohia Hospital Baba Kharak Singh Marg, New Delhi-110001 India rohanmagoon21@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Over the past decades, there have been noteworthy advancements in the cardiac surgical practice that have assisted fast-tracking and enhanced recovery after cardiac surgery (ERACS). With that said, intensive care unit (ICU) readmission in this high-risk patient cohort entails a significant morbidity–mortality burden. As an extension of the same, there has been a heightened emphasis on a comprehensive evaluation of the predisposition to readmission following a primary ICU discharge. However, the variability of the institutional perioperative practices and the research complexities compound our understanding of this heterogeneous outcome of readmission, which is intricately linked to both patient and organizational factors. Moreover, a discussion on ICU readmission in the recent times can only be rendered comprehensive when staged in close conjunction to the fast-tracking practices in cardiac surgery. From a more positive probing of the matter, a preventative outlook can likely mitigate a part of the larger problem of ICU readmission. Herein, focused cardiac prehabilitation programs can play a potential role given the emerging literature on the positive impact of the former on the most relevant readmission causes. Therefore, the index review article aims to address the subject of cardiac surgical ICU readmission, highlighting the magnitude and burden, the causes and risk-factors, and the research complexities alongside deliberating the topic in the present-day context of ERACS and cardiac prehabilitation.
Keywords
cardiac surgery
intensive care unit
prehabilitation
readmission
research complexities
Introduction
Needless to say, we have come a long way and continue to march ahead in our endeavors to refine the anesthesia-perfusion-surgical conduct aimed at ameliorating the perioperative morbidity and mortality in the cardiac surgical practice. Withstanding the same, fast-tracking and enhanced recovery after cardiac surgery (ERACS) are becoming increasingly conceivable in the present times.1, 2, 3 As we embrace the ever-growing opportunities to fast-track following cardiac surgery, there is a concurrent need to holistically assess the research landscape of a closely related and equally (if, not more) important phenomenon of intensive care unit (ICU) readmission in cardiac surgical subset following a primary discharge.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15
Readmission as an Event is “Far from Benign”
The incidence of ICU readmission in cardiac surgical patients within the course of the index hospitalization ranges from 2 to 8% based on the existing relevant literature.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 ICU readmission entails a protracted length of stay in the hospital and consequent unfavorable clinical outcomes. The in-hospital mortality rates are to the remarkable tune of 11 to 24% for the readmitted patient cohort.4 There are studies to suggest as much as six times escalation in mortality rates and, three to seven times the increase in the length of hospital stay and the length of ICU stay, respectively, in the readmitted patients, in contrast to the nonreadmitted patients.7, 8 The former negatively impacts the morale of the entire perioperative team and more importantly, the resolute of the primary patient caregivers. Moreover, the resultant strain imposed on the health care system can also not be ignored, particularly in the context of an increased cost and resource burden on the already constrained ecosystem of the low- and middle-income countries.4, 5, 7, 8, 9, 10, 11, 12, 13, 14, 15 Bettex and Rudiger adequately elaborate the negative impact of readmission not only on the health system but the family of the patient as well.7
ICU Readmission: Causes and Risk Factors
The causes for ICU readmission in cardiac surgical subset outlined by the various independent research groups have been summarized in Table 1.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 The majority of the ICU readmissions result owing to the underlying respiratory and cardiac reasons. Postoperative pneumonia (hospital-acquired/aspiration), pleural–effusion, poor ventilatory reserve, etc., leading to hypoxemia and respiratory failure necessitating reintubation and mechanical ventilation, account for the major cause of readmission. Cardiovascular decompensation, precipitated by arrhythmias and heart failure, classifies as the second leading cause.13 The need for reoperative interventions, sepsis, and other morbid organ outcomes also contributes to the overall ICU readmission burden (Table 1).4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15
|
• Respiratory failure (a substantial contributing percentage of 34.13–54.9%) |
|
• Cardiac decompensation or cardiovascular instability (principal cause almost in every 4th readmitted patient, with some being post-CPR) |
|
• Reoperative interventions for cardiac tamponade/bleeding (the causative percentage hovers around 6–9.58%)a |
|
• Postoperative renal failure (3–6.6%) |
|
• Sepsis (1.5–3.59%) |
|
• Gastrointestinal complications (2–6%) |
|
• Neurological reasons or an altered mental status (0.5–4.79%) |
|
• Miscellaneous (peripheral ischemia, allergic reactions, embolic occlusion of peripheral vasculature, sternal dehiscence or DSWI, etc.) |
Abbreviations: CPR, cardiopulmonary resuscitation; DSWI, deep sternal wound infections; ICU, intensive care unit.
Simultaneously, there has been an ever-increasing emphasis on evaluating the risk factors responsible for predisposing the discharged patients to the likelihood of being readmitted to the ICU.5 Table 2 enlists the various preoperative patient-related, operative, and the primary ICU stay postoperative factors identified as the ICU readmission risk predictors across the existing cardiac surgical literature.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15
|
Preoperative: |
|
• Pre-existing renal failure (a powerful independent readmission predictor in the literature) |
|
• COPD or chronic lung disease (COPD, in particular entails an elevated risk) |
|
• Previous myocardial infarction |
|
• High EuroSCORE IIa |
|
• Advanced age (mixed literature with age in excess of 70–80 years being implicated) |
|
• Other potential factors adding to the risk (reduced LVEF, ≥NYHA III status, female sex, preoperative arrhythmias, diabetes mellitus, neurological disease, triple vessel disease, morbid obesity, PAD, Parsonett score) |
|
Operative and primary ICU stay postoperative factors: |
|
• Emergency surgery |
|
• Combined CABG + valve surgery (a consistent operative risk-factor with additional risk contributed by redo-surgery, aortic procedure and prolonged ACC and CPB times) |
|
• Post-cardiotomy low cardiac output syndrome (requiring IABP/VAD assistance) |
|
• Prolonged mechanical ventilation > 24 hours (studies also implicate a primary ICU stay > 72 hours as a risk-factor) |
|
• Postoperative arrythmias |
|
• Need of hemofiltration/dialysis |
|
• Pulmonary complications |
|
• Cardiopulmonary resuscitation |
|
• Re-exploration for bleeding |
|
• Postoperative anemia and neurological dysfunction |
|
• Contributing factors, such as ICNARC score, inotropic requirement, fraction of inspired oxygen requirement, respiratory rate, gastrointestinal bleed and graft-infection. |
Abbreviations: ACC, aortic cross-clamp; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; COPD, chronic obstructive pulmonary disease; EuroSCORE, European System for Cardiac Operative Risk Evaluation; IABP, intra-aortic balloon pump; ICNARC score, Intensive Care National Audit & Research Centre Score; ICU, intensive care unit; LVEF, left ventricle ejection fraction; NYHA, New York Heart Association; PAD, peripheral arterial disease; VAD, ventricular assist device.
With the major causes and risk factors being outlined, there are additional factors that may contribute to the overall problem. For instance, the inclusion of an overnight prolonged fasting in the traditional surgical bundle accentuates the propensity to surgical stress and catabolism.16, 17, 18 To make the matter worse, our cardiac patients with advanced age, pre-existing malnutrition, comorbidities, and frailty may be even more predilected owing to a limited cardiopulmonary reserve, leading to an all the more challenging postoperative respiratory rehabilitation.19, 20, 21, 22, 23, 24, 25, 26 The above-mentioned factors usher these high-risk surgical subset into a downward spiral manifesting as impaired healing, decreased immune response, heightened risk of surgical complications, readmission rates, hospital stay, and mortality.22, 24, 26, 27, 28
Cardiac Surgical ICU Readmission: A Complex Research Affair
Independent research groups have developed and internally validated various ICU readmission risk predictive models, emanating from their respective cardiac surgical settings (Table 3).8, 9, 10, 11, 12 However, to date, only the Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease risk predictive model by van Diepen et al has been subjected to an external validation in a cohort of 805 patients with a 4.6% readmission rate.9
|
Risk model |
|
• APPROACH model, van Diepen et al, 2014, developed in a prospective dataset of 10,799 CABG/valvular surgical patients, with a 4.4% readmission rate. Following an internal validation with bootstrapping, the model demonstrated an AUC = 0.809 |
|
Components: age, chronic lung disease, LVEF, single-valve/non-CABG surgery, multivalvular surgery, postoperative cardiac-arrest, pneumonia, pleural-effusion, DSWI, leg-graft harvest site infection, GI-bleeding, and neurologic-complication |
|
• APPROACH model refined, Verma et al, 2019, developed in a prospective cohort of 805 CABG/valvular surgical patients, with a 4.6% readmission rate. A good discrimination with an AUC = 0.7810 |
|
Improvised APPROACH model with additional components: reintubation, tracheostomy, inotrope and oxygen requirements at discharge, HR, and SBP |
|
• BATS model, Magruder et al, 2015, developed in a prospective cohort of 421 CABG/AVR patients, with a 6.6% readmission rate. A good discrimination with an AUC = 0.8111 |
|
Components: female sex, NYHA III/V functional status, urgent or emergent operation, and postoperative renal failure during the index ICU admission |
|
• Li et al model, 2019, developed from retrospective data of 824 valve surgery patients, with a 13.4% readmission rate. A good discrimination with an AUC = 0.8812 |
|
Components: age > 65 years, chronic lung disease, previous cardiac surgery, LVEF < 50%; < 40%, NYHA III/IV status, multiple valve surgery, CPB time > 180 minutes, cardiac-arrest, ARDS, pneumonia, DSWI, and postoperative renal failure |
|
• Thomson et al model, 2018, developed retrospectively from a mixed surgical cohort of 4,869 patients, with a 3.2% readmission rate. They reported a well-calibrated bootstrapped model with a good discrimination, missing details on the statistical performance8 |
|
Components: surgical urgency, diabetes-mellitus, stage 3–5 chronic kidney disease, aortic valve surgery, hypertension, EuroSCORE II, preoperative neurologic disease, ICNRC score, and postoperative anemia |
Abbreviations: APPROACH, Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease; ARDS, acute respiratory distress syndrome; AUC, area under the curve; AVR, aortic valve replacement; BATS, Bounce Back After Transfer; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; DSWI, deep-sternal wound infections; EuroSCORE II, European System for Cardiac Operative Risk Evaluation; GI, gastrointestinal; HR, heart rate; ICNRC, Intensive Care National Audit & Research Centre Score; ICU, intensive care unit; LVEF, left ventricle ejection fraction; NYHA, New York Heart Association; SBP, systolic blood pressure.
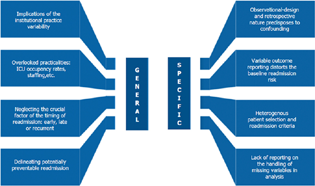
Furthermore, the very recently published systematic review on the topic of cardiac surgical ICU readmission by Kimani et al included a total of 25 ICU readmission studies and 5 readmission risk models.5 Worthwhile to mention, the research group could not inculcate a formal meta-analysis owing to the range of inconsistencies that likely precluded the outcome-pooling across the studies and hence, robust statistical inferences.5 Therefore, it becomes imperative to meticulously consider the intricate nuances of the readmission research.4, 5, 6, 7, 8, 9, 10, 11, 12, 27, 28, 29, 30, 31 Fig. 1 illustrates the general and specific complexities of the ICU readmission research in perioperative cardiac care.
At the same time, the moderate precision with which ICU readmission can be predicted in practice only goes on to interrogate its use as a quality indicator.30 If at all so, the readmissions limited to the first 48 hours of a primary discharge might connote the ICU performance to some extent, as elucidated by Bettex and Rudiger.6, 7
ICU Readmission in the Era of Fast-Tracking
A discussion on ICU readmission would only be complete when staged in close conjunction to the recent multimodal transdisciplinary initiative of ERACS and fast-tracking (an early tracheal extubation within 6 to 10 postoperative hours, an important component of ERACS).1, 2, 3
It is encouraging that the existing literature does not suggest heightened rates of ICU readmission following fast-tracking in cardiac surgery.27, 28, 32 In a series of evaluation of the risk of ICU readmissions while fast-tracking, Kogan et al depicted an overall 3.29% readmission over a 27-month long period of prospectively studying 1,613 cardiac surgical patients. The research group outlined a 47.2, 28.3, and 24.5% readmission rate in the first 24 hours, 24 to 48 hours, and after 48 hours of the primary ICU discharge, respectively.27 Notably, the incidence and risk-pattern in the Kogan et al study were largely in congruence with the overall readmission literature.27 Furthermore, Toraman et al specifically delineated the impact of the nature of operative intervention in their discovery of a much higher 5.5% readmission rate in coronary artery bypass grafting (CABG) + valve surgeries compared with the 1.9% readmission rate in the patients undergoing an isolated CABG being fast-tracked.28
Nonetheless, with the fast-track protocols emerging as the standard of care across cardiac surgical centers, it remains to be highlighted that the practicalities surrounding any intervention aimed at an early ICU discharge should simultaneously consider the possibility of a subsequent clinical deterioration necessitating an unplanned ICU readmission.27 For instance, a systematic review and meta-analysis by Vollam et al that suggest heightened ICU readmission rates in background of an out-of-hours discharge policy in a generalized ICU population are noteworthy from a practical standpoint.33
With the 2016 Cochrane systematic review deciphering the safety of fast-track protocols in patients with low-moderate perioperative risk,32 the detrimental impact of failure to fast-track mandates due consideration amidst ever-growing complexities of the cardiac surgical interventions and the comorbid patient profile.27, 28 This doubtlessly calls for a prudent patient selection.34 As far as the dynamic factors are concerned, Probst et al propose to delay the decision-making on fast-track suitability till the surgical completion, to reduce the eventual rates of fast-track failure.35
Future Directions: Prevention to Prehabilitation
Considering the fact that ICU readmission is a complicated outcome interlinked to both the patient and organizational factors,5 tackling the modifiable risk-factors offers viable opportunities to ameliorate the overall problem. Bettex and Rudiger substantiate the former by elaborating that the readmissions after 48 hours of a primary discharge are associated with the comorbid profile of the patients.6, 7 This brings forth attention to the concept of potentially preventable ICU readmission. However, identifying the same can be peculiarly challenging in the cardiac surgical arena particularly when readmission studies tend to overlook the crucial factor of the timing of readmission as cited in Fig. 1. Therefore, future readmission research should aim at delineating the “true” incidence of this potentially preventable ICU readmission in the perioperative practice.6
Meanwhile, looking at the patient-specific predisposition to ICU readmission in cardiac surgery,4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 cardiac prehabilitation with its beneficial attributes like aerobic-conditioning, respiratory muscle training, lifestyle-modifications, nutritional and mental-health upliftment can likely provide some potential solutions.36, 37, 38, 39, 40 Such a possibility is best seen in the light of the cardiac prehabilitation (a domain aimed at maintaining an adequate physiological-physical-psychosocial status of the patients to effectively mitigate the period of surgical stress) literature demonstrating improved postcardiac surgical functional ability and respiratory mechanics and, a reduced incidence of pulmonary complications like pneumonia, particularly when the respiratory causes contribute significantly to ICU readmission.36, 39, 40, 41
Conclusion
The existing literature implies a substantial morbidity–mortality burden to ICU readmission in cardiac surgical patients. Nevertheless, it is time we comprehend the complexities of ICU readmission better and increasingly focus on the research area from a much-required predictive and a preventative perspective. We are equally hopeful that the adoption of well-designed cardiac prehabilitation programs would be conducive to the overall aim of curtailing the burden of ICU readmission in cardiac surgical practice.
Conflict of Interest
None declared.
References
- Multimodal analgesia in paving the way for enhanced recovery after cardiac surgery. Rev Bras Cir Cardiovasc. 2022;••• [ahead of print, October 18]
- [CrossRef] [Google Scholar]
- Guidelines for perioperative care in cardiac surgery: enhanced recovery after surgery society recommendations. JAMA Surg. 2019;154(8):755-766.
- [Google Scholar]
- Enhanced recovery after cardiac surgery: is it just about putting the bundles together? Ann Card Anaesth. 2021;24(2):276-278.
- [Google Scholar]
- Perioperative risk factors for intensive care unit readmissions and mortality after cardiac surgery. J Cardiothorac Vasc Anesth. 2022;36:2339-2343. (8 Pt A):
- [Google Scholar]
- Predicting readmission to intensive care after cardiac surgery within index hospitalization: a systematic review. J Cardiothorac Vasc Anesth. 2021;35(7):2166-2179.
- [Google Scholar]
- Readmission to the cardiac surgery intensive care unit. J Cardiothorac Vasc Anesth. 2022;36(4):1223.
- [Google Scholar]
- Length of ICU stay after cardiac surgery: too long or too short? J Cardiothorac Vasc Anesth. 2018;32(6):2692-2693.
- [Google Scholar]
- Readmission to the intensive care unit following cardiac surgery: a derived and validated risk prediction model in 4,869 patients. J Cardiothorac Vasc Anesth. 2018;32(6):2685-2691.
- [Google Scholar]
- Predicting cardiovascular intensive care unit readmission after cardiac surgery: derivation and validation of the Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease (APPROACH) cardiovascular intensive care unit clinical prediction model from a registry cohort of 10,799 surgical cases. Crit Care. 2014;18(6):651.
- [Google Scholar]
- Prospective validation and refinement of the APPROACH cardiovascular surgical intensive care unit readmission score. J Crit Care. 2019;54:117-121.
- [Google Scholar]
- A predictive model and risk score for unplanned cardiac surgery intensive care unit readmissions. J Card Surg. 2015;30(9):685-690.
- [Google Scholar]
- Analysis of risk factors and establishment of a risk prediction model for cardiothoracic surgical intensive care unit readmission after heart valve surgery in China: a single-center study. Heart Lung. 2019;48(1):61-68.
- [Google Scholar]
- The predictors and outcome of recidivism in cardiac ICUs. Eur J Cardiothorac Surg. 2005;27(3):508-511.
- [Google Scholar]
- Predictors and outcome of ICU readmission after cardiac surgery. Thorac Cardiovasc Surg. 2009;57(7):391-394.
- [Google Scholar]
- ICU readmission after cardiac surgery-still a matter of concern? Thorac Cardiovasc Surg. 2020;68(5):384-388.
- [Google Scholar]
- Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28(8):556-569.
- [Google Scholar]
- Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Anesthesiology. 2017;126(3):376-393.
- [Google Scholar]
- Preoperative fasting guidelines: why are we not following them? The time to act is NOW. Anesth Analg. 2017;124(4):1041-1043.
- [Google Scholar]
- Aerobic reserve and physical functional performance in older adults. Age Ageing. 2008;37(4):384-389.
- [Google Scholar]
- Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16(1):157.
- [Google Scholar]
- EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr. 2008;27(3):340-349.
- [Google Scholar]
- The nutritional strategy: four questions predict morbidity, mortality and health care costs. Clin Nutr. 2014;33(4):634-641.
- [Google Scholar]
- Does the prognostic nutritional index have a predictive role in the outcomes of adult cardiac surgery? J Thorac Cardiovasc Surg. 2020;160(1):145-153.e3.
- [Google Scholar]
- Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156.
- [Google Scholar]
- Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet. 2018;391:2631-2640. (10140):
- [Google Scholar]
- Nutritional assessment: comparison of clinical assessment and objective variables for the prediction of length of hospital stay and readmission. Am J Clin Nutr. 2015;101(5):956-965.
- [Google Scholar]
- Readmission to the intensive care unit after “fast-track” cardiac surgery: risk factors and outcomes. Ann Thorac Surg. 2003;76(2):503-507.
- [Google Scholar]
- Readmission to the intensive care unit after fast-track cardiac surgery: an analysis of risk factors and outcome according to the type of operation. Heart Surg Forum. 2010;13(4):E212-E217.
- [Google Scholar]
- Hospital readmissions after cardiac surgery: is it a game worth playing? J Thorac Cardiovasc Surg. 2015;149(3):858.
- [Google Scholar]
- Implications of practice variability: comment. Comment Anesthesiology. 2020;133(4):943-944.
- [Google Scholar]
- Fast-track cardiac care for adult cardiac surgical patients. Cochrane Database Syst Rev. 2016;9(9):CD003587.
- [Google Scholar]
- Out-of-hours discharge from intensive care, in-hospital mortality and intensive care readmission rates: a systematic review and meta-analysis. Intensive Care Med. 2018;44(7):1115-1129.
- [Google Scholar]
- A specialized post anaesthetic care unit improves fast-track management in cardiac surgery: a prospective randomized trial. Crit Care. 2014;18(4):468.
- [Google Scholar]
- Mind-body-soul intervention: a cardiac surgical prehabilitation program. J Anaesthesiol Clin Pharmacol. 2022;38(2):322-323.
- [Google Scholar]
- Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2016;2016(1):CD001800.
- [Google Scholar]
- Feasibility of preoperative inspiratory muscle training in patients undergoing coronary artery bypass surgery with a high risk of postoperative pulmonary complications: a randomized controlled pilot study. Clin Rehabil. 2006;20(11):949-959.
- [Google Scholar]
- Home-based preoperative rehabilitation (prehab) to improve physical function and reduce hospital length of stay for frail patients undergoing coronary artery bypass graft and valve surgery. J Cardiothorac Surg. 2017;12(1):91.
- [Google Scholar]
- Incentive spirometry for preventing pulmonary complications after coronary artery bypass graft. Cochrane Database Syst Rev. 2012;2012(9):CD004466.
- [Google Scholar]