Translate this page into:
Heart–Lung Interaction
Yatin Mehta, MD, MNAMS, FRCA, FAMS, FIACTA, FICCM FTEE Medanta Institute of Critical Care and Anesthesiology Medanta-The Medicity, Sector-38, Gurugram 122001, Haryana India yatinmehta@hotmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Mechanical interplay between respiratory and cardiac systems was first recognized about 300 years ago when an English physiologist Stephen Hales observed that the level of the blood column in a glass tube inserted into the carotid artery of a horse varied cyclically with respiration.
Heart and lung share the same intrathoracic space, and mechanically this configuration is akin to pump within a pump. As a result, intrathoracic pressure (ITP) and volume changes during respiratory cycle affect the performance of heart. Besides direct mechanical effects, lung and heart interplay also involves certain neurally and humorally mediated phenomena. Taken together, these dynamics constitute heart–lung interaction. Knowledge of heart–lung interaction is especially useful while dealing with critically sick patients on ventilator, because not only are such patients more vulnerable to hemodynamic instability induced by ventilator, but it also forms the basis of functional hemodynamic monitoring.
Keywords
heart
interaction
lung
Introduction
Mechanical interplay between respiratory and cardiac system was first recognized about 300 years ago when an English physiologist Stephen Hales observed that the level of the blood column in a glass tube inserted into the carotid artery of a horse varied cyclically with respiration.1
Heart and lung share the same intrathoracic space, and mechanically this configuration is akin to pump within a pump.2 As a result, intrathoracic pressure (ITP) and volume changes during respiratory cycle affect the performance of the heart. Besides direct mechanical effects, lung and heart interplay also involves certain neurally and humoral mediated phenomena. Taken together, these dynamics constitute heart–lung interaction. Knowledge of heart–lung interaction is especially useful while dealing with critically sick patients on ventilator, because not only are such patients more vulnerable to hemodynamic instability induced by ventilator, but it also forms the basis of functional hemodynamic monitoring.3, 4
Factors Governing Stroke Volume
Stroke volume (SV) is the amount of blood ejected by a ventricle into circulation with each heartbeat.5 SV depends on preload, contractility, and afterload. Under the limit, preload and contractility have positive effect and afterload has negative effect on SV.
Right Ventricular Preload
Preload is the end-diastolic volume (EDV). It depends on venous return (VR) that in turn depends on the gradient for filling up of the right atrium (RA) and resistance to VR. Gradient for VR is equal to the difference between mean systemic venous pressure (MSVP) and the right atrial pressure (RAP) (MSVP − RAP).6, 7 Elastic recoil of veins generates some positive pressure in venous blood that is called MSVP. Increase in intravascular volume or venous tone can increase MSVP. Usually RAP during diastole is approximately equal to and reflects ITP. When ITP increases as during mechanical inspiration, the gradient decreases and so does the preload. Opposite happens during spontaneous inspiration when the gradient increases due to decreased ITP leading to increased preload.8
Decreasing ITP can increase the VR up to a limit only, and beyond a point, the large vessels collapse at the entry to the thorax due to negative ITP limiting any further increase in VR. This is called critical closing pressure of the veins entering the thorax.9 Because ITP changes during each breath, VR gradient, and therefore the VR preload, varies during expiration and inspiration.
In volume-replete state, the decrease in VR induced by increased ITP is mitigated by the fact that increased ITP as seen, for example, while applying positive end-expiratory pressure (PEEP), not only increases RA pressure but also causes increased MSVP by translocation of blood from pulmonary to systemic circulation and descent of diaphragm resulting in increased intra-abdominal pressure and compression of intra-abdominal capacitance vessels (Fig. 1). In volume-deficient state, MSVP fails to rise adequately and VR may fall drastically. In a normal heart, PEEP reduces cardiac output (CO) to some extent in normovolemic and drastically in hypovolemia due to its effect on VR. In a failing heart, PEEP usually improves CO in part by reducing preload besides reducing transmural pressure.

- Effect of increase in intrathoracic pressure on venous return in volume replete state. ITP, intrathoracic pressure; MSVP, mean systemic venous pressure.
Left Ventricular Preload
Left ventricular (LV) preload averaged over time is essentially equal to right ventricular (RV) output. Because RV preload and therefore RV SV vary during respiratory cycle, LV preload follows the pattern with a lag time equal to time taken by blood to transit pulmonary circulation.10 During mechanical inspiration, as the ITP rises while the VR to RV decreases, the VR to LV increases due to squeezing of blood forward into the LV. Subsequently, reduced RV preload manifests as reduced RV SV that reaches the LV after a few heartbeats, resulting in reduced LV preload.
Contractility and Starling's Law
Starling's law of the heart states that within limits, the SV of the heart increases in response to an increase in the volume of blood in the ventricles, before contraction (EDV), when all other factors remain constant.11
When a muscle fiber is at its optimal length, it can develop maximum isometric active tension. In a normal resting state, the muscle fibers of the ventricles are at less than the optimal length of contraction. This implicates that if the ventricular muscle fibers get stretched a little due to increased preload, they will contract more strongly to achieve a higher SV.11 Graph charting SV against EDV is of sigmoid shape. Maximal response to change in EDV is seen during the steep part of the graph. This forms the basis to define fluid responsive state. If there is wide enough SV variation (SVV) in response to variation in preload, it indicates a fluid responsive state. As noted previously, due to variation in preload during each breath, SVV is expected.
Afterload
Afterload is the pressure against which the heart must work to eject blood during systole.12 Afterload is often expressed as ventricular wall stress (σ).

where P is ventricular pressure; r is ventricular radius; and h is wall thickness.
Afterload increases by increase in transmural pressure and vascular resistance.
Concept of Transmural Pressure
Heart is situated in juxtacardiac fossa surrounded by the lungs, and the whole apparatus is situated within the thoracic cage. The immediate pressure surrounding the heart is pericardial pressure. Usually in absence of pericardial disease, pericardial pressure is assumed to be equal to pleural pressure.12 Pleural pressure in turn varies as per phase of respiration and is affected by whether the respiration is mechanical or spontaneous among other factors.13 Most commonly, it is the intracardiac pressures such as RAP or left atrial pressure, which are readily available at bedside. It should be kept in mind that these pressures are measured in reference to atmospheric pressure and not the pressure that surrounds the heart. Transmural pressure of the heart is the actual distending pressure of the cardiac chamber5 and is the actual “working pressure” against which the walls of heart chambers have to generate by contracting (Figs. 2 3). Transmural pressure = intracavitary pressure − surrounding pressure. In simple words, if surrounding pressure of the heart is negative, during the systole, ventricle has to overcome the extra load of outward distending forces on its wall due to surrounding negative pressure. Similarly, if the surrounding pressure is positive, it compresses the ventricles from outside, thereby aiding in systole.

- Intrathoracic pressure (P1) is the pressure in inner chamber with respect to atmosphere, P2 is the pressure in outer chamber with respect to atmosphere. Transmural pressure is the net pressure experienced by the inner chamber walls. Tm = P1-P2.

- If the intrathoracic pressure is −20 mm Hg, to generate 80 mm Hg pressure in aorta, ventricles will have to contract strongly enough to overcome −20 mm Hg outward pulling pressure and thus effectively will have to work equivalently to generate 100 mm Hg pressure. On the other hand, if intrathoracic pressure were +20 mm Hg, ventricles will have to generate only 60 mm Hg pressure 80 mm Hg in aorta. LV, left ventricle.
Determinants of Left Ventricular Afterload
As already discussed, afterload is determined by vascular resistance and transmural pressure. Because SVR does not fluctuate remarkably during respiratory cycle, it is the fluctuations in transmural pressure, which majorly decide the variations in LV afterload. If the ITP is positive (as during mechanical inspiration), it compresses the ventricle and aids in ejection. Transmural pressure becomes lower; however, in case of negative ITP (as during spontaneous inspiration) (transmural pressure = intraventricular − ITP), the ventricle needs to contract more strongly to generate higher transmural pressure. During mechanical ventilation, ITP rises leading to decrease in transmural pressure for the LV.10, 14, 15, 16, 17
Determinant of Right Ventricular Afterload
Because both RV and pulmonary vasculature are exposed to similar swings in ITP, unless there is extreme rise in ITP, it is the variations in pulmonary vascular resistance (PVR) that play a more dominant role in deciding afterload. PVR varies significantly with lung volume as described next.18, 19
Pulmonary Vascular Resistance Variation with Lung Volume
From point of view of effect of lung volume on vascular cross-sectional area, pulmonary vasculature can be divided into extra-alveolar vessels and intra-alveolar vessels. The former consists of vessels running in the lung interstitium, and the latter, capillaries exposed in alveolar space. When the lung volume increases the cross-sectional area of extra-alveolar vessels, it decreases in alveolar vessels and vice versa20 (Fig. 4).

- Physical effect of lung volume on pulmonary vasculature diameter. FRC, functional residual volume.
Therefore, at low lung volume, extra-alveolar vessels are compressed, and at high lung volume, alveolar vessels are compressed. As a result of this PVR, increase in both high and low lung volume is minimum at functional residual volume. In addition to mechanical effects, at low lung volume during hypoventilation, alveolar hypoxia and resorption atelectasis may happen resulting in hypoxic vasoconstriction of pulmonary vessels. This adds to PVR during low lung volume (Fig. 5).

- Graphical representation of effect of lung volume on pulmonary vascular resistance. FRC, functional residual volume.
Acute Cor Pulmonale
Right ventricle is adapted to low-resistance pulmonary vasculature and does not work efficiently at high pressure.21 Acute rise in afterload is poorly tolerated by the RV as compared with the LV, which possesses much higher contractile reserves.14
Such acute rise may be seen in air trapping, acute respiratory distress syndrome (ARDS) with lung derecruitment and hypoxic vasoconstriction, pulmonary embolism, etc. In this situation, RV failure happens leading to increased RV EDV. Increased RV EDV may lead to fall in LV SV due to phenomenon of ventricular interdependence, as described next.
Phenomenon of Ventricular Interdependence
Both ventricles share fixed space within pericardium. Because LV and RV share fixed space within pericardium and a common septum, the diastolic pressure of one ventricle directly affects the diastolic filling of the other.14 When RV volume is increased, LV filling declines. This leads to what is called phenomenon of ventricular interdependency wherein rise in RV afterload leads to increased RV EDV and therefore decreased EDV or preload of the LV; this subsequently leads to decreased LV SV (Fig. 6). This phenomenon can be seen in conditions with RV afterload elevation (e.g., pulmonary embolism, pulmonary hypertension) or as a cyclical respiratory physiologic phenomenon that can be aggravated by disease (e.g., pericardial effusion) or mechanical ventilation.21

- Ventricular interdependence. LV, left ventricle; PVR, pulmonary vascular resistance; RV, right ventricle.
Consolidated Effect of Spontaneous Respiration
During inspiration, ITP decreases and therefore VR increases, and at the same time, RV afterload decreases due fall in PVR. This leads to increased RV output that will take time to traverse the pulmonary circulation before it becomes LV preload. At the same time, LV preload decreases due to immediate pooling of blood in pulmonary circulation. Also, LV afterload and transmural pressure increase by negative ITP. This results in decreased LV SV during spontaneous inspiration and can be seen as decreased systolic and pulse pressure on arterial trace at bedside. Subsequently by the time of expiration, the increased quantum of RV output generated during inspiration reaches the LV, and this manifests as increased LV SV and increased systolic and pulse pressure on arterial pressure trace22 (Fig. 7).
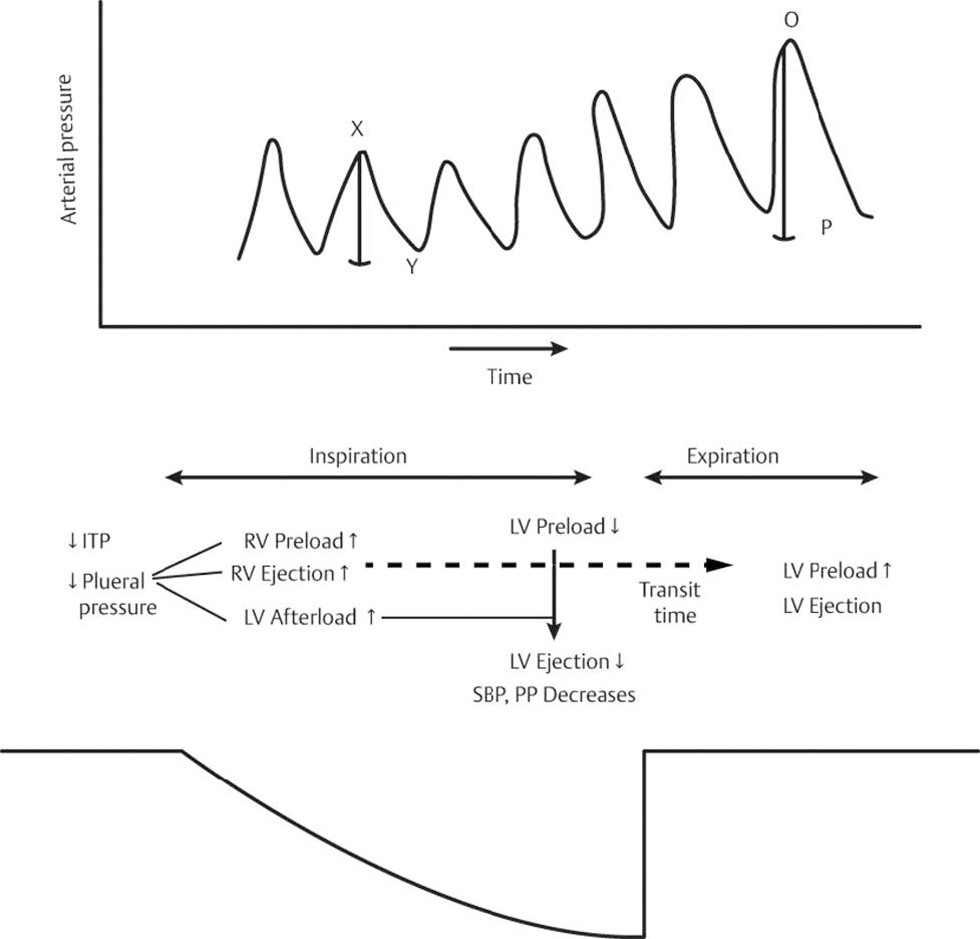
- Heart lung interaction: effects of spontaneous respiration. ITP, intrathoracic pressure; LV, left ventricle; O-P, increased pulse pressure on arterial pressure trace during spontaneous expiration; RV, right ventricle; SBP, systolic blood pressure; x-y, reduced pulse pressure on arterial pressure trace during spontaneous inspiration.
Consolidated Effect of Mechanical Ventilation
The consolidated effect of mechanical ventilation in normoor hypovolemia is as follows. During inspiration, ITP rises and therefore VR decreases, and at the same time, RV afterload increases due to rise in PVR. This leads to decreased RV output that will take time to traverse the pulmonary circulation before it becomes LV preload. At the same time, LV preload increases due to immediate squeezing of blood out of pulmonary circulation by increased ITP also LV afterload and transmural pressure is decreased by positive ITP. This results in increased LV SV during positive-pressure inspiration and can be seen as increased systolic and pulse pressure on arterial trace at bedside. Subsequently by the time of expiration, the decreased quantum of RV output generated during inspiration reaches the LV, and this manifests as decreased LV SV and decreased systolic and pulse pressure on arterial pressure trace22 (Fig. 8).

- Heart-lung interactions: effects of mechanical ventilation. Increased intrathoracic pressure due to positive-pressure ventilation increases right ventricular (RV) afterload, whereas RV preload is decreased due to the decreased venous return, at the same time left ventricular (LV) preload is transiently increased due to a “squeezing” effect from pulmonary vasculature into the left atrium. Ultimately, however, the decrease in RV filling leads to decreased LV filling and output. AB, widened pulse pressure on arterial trace during mechanical inspiration; CD, reduced pulse pressure on arterial trace during expiration on ventilator; LV, left ventricle; RV, right ventricle. (Adapted from Michard and Teboul, 2000.)
Clinical Implications
Heart–lung interaction has been exploited in use of dynamic hemodynamic indices.
Pulse Pressure Variation and Its Variants (PPV and SVV) During mechanical ventilation, ITP changes cyclically and causes similar changes in RAP. RAP rises during mechanical inspiration and falls with exhalation. As a result, VR and RV SV drop with inspiration and rise with exhalation. This variation of RV SV causes with a time delay—the pulmonary transit time—variation in LV preload and SV. The SVV is maximally seen if the heart works on the steep part of starling curve; that is, it is responsive to fluid administration. This SVV results in difference between systolic arterial pressure (SAP) and diastolic arterial pressure (DAP) in the peripheral arterial pressure curve to rise during inspiration and fall in expiration.
PPV ≥ 12% is strongly associated with fluid responsiveness in septic patients with positive predictive value of > 12%.22
SVV is based on the same principle as PPV. SVV > 10% is associated with fluid responsiveness (SVV).23 24
Conflict of Interest
None.
References
- Vascular access: an historical perspective from Sir William Harvey to the 1956 Nobel Prize to André F. J Vasc Access. 2012;13(2):137-144.
- [Google Scholar]
- Effect of Mechanical Ventilation on Heart-Lung Interactions Principles and Practice of Mechanical Ventilation. (3rd ed.). New York, NY: McGraw Hill; 2012.
- [Google Scholar]
- Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med. 2001;29(8):1551-1555.
- [Google Scholar]
- Clinical death and the measurement of stressed vascular volume. Crit Care Med. 1998;26(6):1061-1064.
- [Google Scholar]
- Reply to “Letter to the Editor: Why Persist in the Fallacy That Mean Systemic Pressure Drives Venous Return?.”. Am J Physiol Heart Circ Physiol. 311 5
- [Google Scholar]
- Levosimendan (OR-1259), a myofilament calcium sensitizer, enhances myocardial contractility but does not alter isovolumic relaxation in conscious and anesthetized dogs. Anesthesiology. 1994;81(4):974-987.
- [Google Scholar]
- Venous return at various right atrial pressures and the normal venous return curve. Am J Physiol. 1957;189(3):609-615.
- [Google Scholar]
- RV filling modulates LV function by direct ventricular interaction during mechanical ventilation. Am J Physiol Heart Circ Physiol. 2005;289(2):H549-H557.
- [Google Scholar]
- Vander's Human Physiology: The Mechanisms of Body Function. (14th ed.). New York, NY: McGraw-Hill Education; 2016.
- [Google Scholar]
- Hemodynamic consequences of heart-lung interactions. J Intensive Care Med. 2003;18(2):92-99.
- [Google Scholar]
- Positive pleural pressure decreases coronary perfusion. Am J Physiol. 1990;258(3):H814-H820. Pt 2
- [Google Scholar]
- Using ventilator and cardiovascular graphics in the patient who is hemodynamically unstable. Respir Care. 2005;50(2):262-274. discussion 274
- [Google Scholar]
- Right ventricular function and positive pressure ventilation in clinical practice: from hemodynamic subsets to respirator settings. Intensive Care Med. 2003;29(9):1426-1434.
- [Google Scholar]
- Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol (1985). 1999;87(5):1644-1650.
- [Google Scholar]
- Extra-alveolar vessels and intra-alveolar vessels: two cases of pulmonary arteriovenous fistula [author's transl] Nihon Kyobu Shikkan Gakkai Zasshi. 1982;20(2):230-238.
- [Google Scholar]
- Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev. 2008;4(1):49-59.
- [Google Scholar]
- On the mechanical factors which determine the output of the ventricles. J Physiol. 1914;48(5):357-379.
- [Google Scholar]
- Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162(1):134-138.
- [Google Scholar]
- Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119(3):867-873.
- [Google Scholar]






