Translate this page into:
Extracorporeal Membrane Oxygenation Carbon Dioxide Removal
*Corresponding author: Yatin Mehta, Department of ECMO, RVCC, Mumbai, Maharashtra. yatinmehta@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Oza P, Goyal V, Mehta Y, Kanchi M, Singh R, Kapoor P. Extracorporeal membrane oxygenation carbon dioxide removal. J Card Crit Care TSS 2023;7:6-11.
Abstract
Protective lung ventilation is the mainstay ventilation strategy for patients on extracorporeal membrane oxygenation (ECMO), as prolonged mechanical ventilation increases morbidity and mortality; the technicalities of ventilation with ECMO have evolved in the last decade. ECMO on the other end of the spectrum is a complete or total extracorporeal support, which supplies complete physiological blood gas exchanges, normally performed by the native lungs and thus is capable of delivering oxygen (O2) and removing CO equal to the metabolic needs of the patient, it requires higher flows, is more complex, and uses bigger cannulas, higher dose of heparin and higher blood volume for priming. This review describes in detail carbon dioxide removal on ECMO.
Keywords
ECMO
Carbon-dioxide removal
Protective lung ventilation
low flows on ECMO
INTRODUCTION
Protective lung ventilation is the mainstay ventilation strategy for patients on extracorporeal membrane oxygenation (ECMO), as prolonged mechanical ventilation increases morbidity and mortality; the technicalities of ventilation with ECMO have evolved in the last decade. Ventilator-associated lung injury augments multiorgan failure on ECMO and this is a dreaded scenario. The National Institute of Health showed in the year 2000 that ventilating patients on ECMO with a tidal volume (TV) of 6 mL/kg with a maximum end-inspiratory plateau pressure of 30 cm H2O instead of 12 mL/kg VT decreased the mortality by 80%.[1] However, even after this scenario, there was a generalized under use of protective ventilatory strategies on ECMO because of the presence of “hypercapnia.” Even with low TVs of ventilation, lung hyperinflation still occurred in about 30% of ARDS patients even with correct ventilation of ARDS net strategy. The idea of partial support ventilation has been proposed in the 1970’s by Kolobow et al.[2,3] and Gattinioni et al.[4] The latter suggested that applying only a few ventilator breaths at low volumes and low peak inspiratory pressures (lung rest) could prevent damage to the compromised lungs. These authors used renal replacement therapy by adding to the circuit an oxygenator and proposed the concept of removing “only a portion of carbon dioxide (CO2) production” to allow less traumatic ventilator settings. Thus “disconnecting” oxygenation from cardiac output (CO) clearance may minimize or prevent ventilator-induced lung injury is the basic concept of extracorporeal CO2 removal.
ECMO PHYSIOLOGY FOR EXTRACORPOREAL CARBON DIOXIDE REMOVAL (ECCO2R)
Renal replacement is the simplest mode of providing mechanical renal support as it provides very low blood flow. CO2 extraction at this low flow with low primer volumes and using a coaxial catheter is removed but at very low levels.[5,6] ECMO on the other end of the spectrum is a complete or total extracorporeal support, which supplies complete physiological blood gas exchanges, normally performed by the native lungs and thus is capable of delivering oxygen (O2) and removing CO equal to the metabolic needs of the patient, it requires higher flows, is more complex, and uses bigger cannulas, [Table 1] higher dose of heparin and higher blood volume for priming.
| Renal support (CVVH) VV | Partial extracorporeal support (ECCO2R) | Total Extracorporeal support (ECMO) | ||||
|---|---|---|---|---|---|---|
| VV | AV | VV | AV | |||
| O2 transfer (mL/min) | - | 10 | 20-60 | 140–340 | 140–340 | 340 |
| CO2 extraction (% of baseline) | - | 25 | 50 | >50 | >50 | >50 |
| Vascular access | VV shunt VV shunt | VV shunt | VV femoral shunt | VV shunt | AV Shunt | |
| (F) | Double-lumen | Double-lumen | V: 15 | Output 15–21 | V: 16 | |
| Needs for heparin (IU/min) | 4–12 | 4–18 | 3.5–10 | 10–20 | 10–20 | |
| Approximate priming volume of circuit | 2,000 mL | 350 mL | 500 mL | |||
ECCO2R: Extracorporeal carbon dioxide removal, ECMO: Extracorporeal membrane oxygenation, VV: Venovenous, AV: Arteriovenous
INTERMEDIATE COMPLEXITY MECHANICAL SUPPORT
The intermediate complexity of mechanical support is partial extracorporeal support (ECCO2R) which uses a 14 fr coaxial catheter to allow a blood flow of 0.3–0.5 L/min, using a roller occlusive pump which is designed to decrease hemolysis, the flow source is at a rate 6–8 L/min. Some devices include a hemofilter with the oxygenator, to allow the extraction of plasmatic water which is then infused in the circuit to prevent blood clotting.[7] This reduces the hematocrit. Instead a centrifugal pump is an alternative, which creates a radial flow through an annual fiber oxygenater. Both can be used in venovenous (VV) ECCO2R systems and remove up to 25% of CO2 and transfer maximally 10 mL/min of O2. Low dose of heparin (4–18 L/min) is used to avoid clotting.[8]
DESCRIPTION OF ECCO2R SYSTEMS
Early VV-ECCO2R
In 1976, Kolobow et al. began to explore the possibility of treating severe respiratory failure using low frequency positive pressure ventilation alongside ECCO2R (LFPPVECCO2R) and in 1977, they demonstrated that O2 uptake and CO2 removal could be dissociated in sheep. The circuits that they used were effectively VV ECMO circuits run at lower flow rates.They required a high level of anticoagulation and two surgically inserted large bore cannula, so bleeding was a major complication with mean daily transfusion requirements reported to be around 3.7–4.0 liters. The initial clinical trial of LFPPV ECCO2R showed promise but a subsequent randomized and controlled trial failed to demonstrate a survival benefit.
ARTERIOVENOUS (AV)-ECCO2R
The concept of arterial venous pressure difference driving an ECCO2R system was considered at an early stage in ECCO2R development, but it only became a feasible treatment option with the advent of low resistance (10 mm Hg/2 L/min) polymethylpentene membranes. The first clinical study of AVECCO2R commenced in 1975 and the first commercially available AV-ECCO R system was released in 2002 (iLA Membran ventilator, Novalung GmbH, Hechingen, Germany). AV-ECCO R is by far the most widely used ECCO2R technique to date [Figure 1].
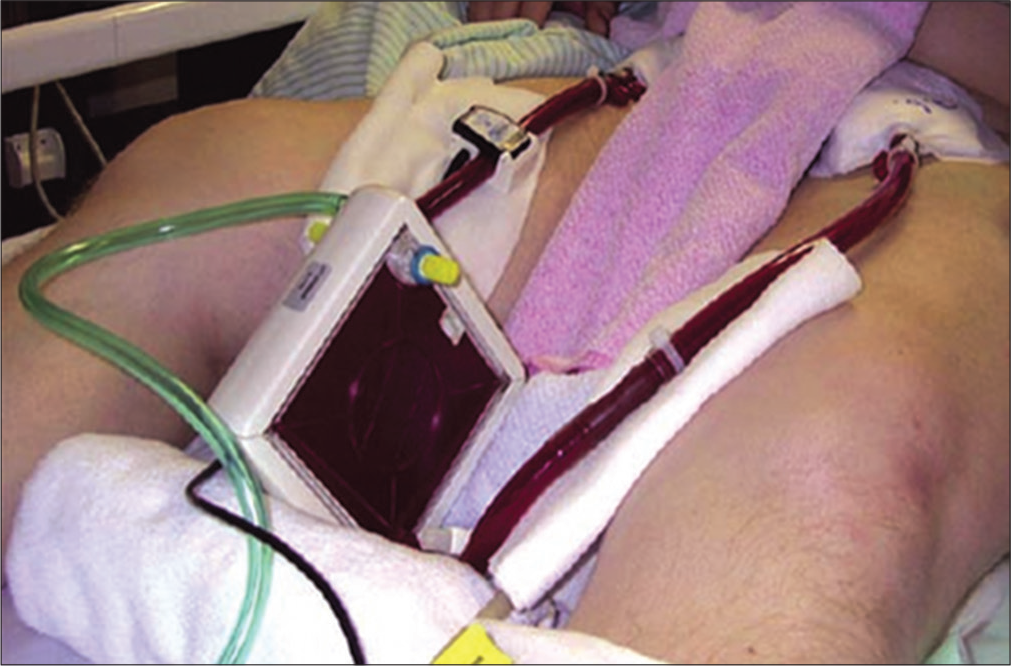
- Arteriovenous-extracorporeal carbon dioxide removal (Novalung iLA).
METHODOLOGY OF AV-ECCO2 SYSTEM
AV-ECCO2R systems involve the insertion of a gas exchange membrane across an AV shunt. The gas exchange membrane is connected to O2 which acts as a “sweep gas” to remove CO2 that has diffused out of the patient’s blood. The flow rate of O2 is increased in a step-wise fashion up to a maximum of 12 L/min. The shunt is usually created between the femoral artery and the contralateral femoral vein using a percutaneously inserted cannula. If necessary, unilateral placement is possible, as is pronging a patient with the device in situ. A well-designed study by Muller et al.[6] demonstrated that:
The primary determinants of blood flow through the system are: the dimensions of the cannula (in accordance with the Hagen Poisseuille equation), the AV pressure gradient (rather than CO), and the resistance of the membrane.
The rate of CO2 removal depends on: Blood flow through the system, sweep gas flow, the partial pressure of CO2 (PaCO2) in the blood supplying the device, and the properties of the membrane (in accordance with Fick’s law of diffusion).
LIMB ISCHEMIA IS DREADED COMPLICATION ON AV-ECCO2R
The most significant complication of AV-ECCO2R is limb ischemia caused by mechanical obstruction to arterial flow and the “steal” effect caused by blood being diverted through the artificially created shunt. The risk of ischemia is therefore related to the diameter of the arterial cannula. Reducing the diameter of the cannula has to be balanced against the effect on flow, but Novalung has reduced the recommended gauge of the arterial cannula to 13F (if the internal arterial diameter is 5.2–6 mm) or 15F (if the internal arterial diameter is more than 6 mm). It is also recommended that ultrasound is used to ensure that the arterial lumen is at least 1.5 times the size of the arterial cannula.
MODERN VV-ECCO2R
The most recent development in ECCO2R technology has been a return to VV-ECCO2R systems. However, modern VV ECCO2R systems are very different from the VV systems used in the 80s and 90s [Table 2]. Their configuration is similar to that of a hemofilter, with a double lumen venous cannula connected to a VV circuit driven by a pump. This removes the potential for complications related to an arterial cannula and means that the system is not dependent on the patient’s heart to generate a pressure gradient. However, the pumped system has the potential to trigger more of an inflammatory response and to cause more hemolysis than a pumpless system. There are currently two commercially available VV-ECCO2R systems, each with their own characteristics.
| Early VV-ECCO2R | AV-ECCO2R | Modern VV-ECCO2R | |
|---|---|---|---|
| Vascular access | Surgically inserted large bore venous cannula (x2) | Percutaneous arterial (13–15F) and venous (15–17F) cannula | Percutaneous double lumen venous cannula. Decap: 14F iLA Activve: 18–24 F |
| Approximate priming volume of circuit | 2,000 mL | 350 mL | 500 mL |
| Membrane properties | Sillicon 8 m2 | PMP 1.3 m2 | PMP Decap: −0.33 m2 iLA Activve: −1.3 m2 |
| Approximate flow rates | 2–4 L/min | 1–2 L/min | Decap<0.5 L/min iLA Activve variable (0.5–4.5 L/min) |
| Target APTR | 2–2.5 | 1.5–2 | 1.1–1.7 |
| Other comments | Uncertain benefits and large amounts of blood loss | Significant complications related to arterial cannula | Lack of supporting evidence at present (only recently introduced) |
ECCO2R: Extracorporeal carbon dioxide removal
TWO CURRENTLY AVAILABLE VV-ECCO2R SYSTEMS ARE USED ACTIVELY
Decap (Hemodec, Salerno, Italy) was the first modern VV-ECCO2R system to be produced. It is a roller-ball pumped system that runs at flow rates of up to 400 mL/min. The circuit also contains a hemofilter, which according to the manufacturers “allows complete control over the lung kidney interaction in multiple organ failure patients.” An initial animal study in 2006 demonstrated no adverse events and a 20% of reduction in CO2 using a flow rate of around 5% of the CO.[9] Its use has since been reported in two small clinical studies[10,11] and a case report[12] with promising results
iLA Activve (Novalung, Germany): The other modern VV system available is the iLA Activve (Novalung, Germany) which has the capacity to run at low or high flow rates (0.5–4.5 L/min). Its use has yet to be reported in the literature but there are plans for a randomized and controlled trial in 2013 patients (“REST” trial). Nova lung promotes the iLA Active as “the all-rounder: The VV system that covers the full range of respiratory support from highly effective CO2 elimination to complete oxygenation.” It uses a centrifugal pump which in theory should cause less hemolysis than a roller head pump, although hemolysis has not been reported as a problem with the Decap system. Another significant difference between these two VV systems is the size of double lumen venous catheter required:
The Decap system can be used with a 14F catheter, although in the study by Terragni et al.[10] The 14F dual lumen catheter had to be replaced by two 8F single lumen catheters in 3/10 patients to achieve flow rates of 400 mL/min. Novalung produces three sizes of double lumen catheter for the iLA Activve ranging from 18F (optimal flow range 0.6–1 L/min) to 24F (optimal flow range 1.25–2 L/min). This suggests that two venous catheters are required to run flow rates above 2 L/min. As a comparison, the double lumen catheters that are used for hemofiltration are usually 11-14F.
MECHANISM OF ECCO2R PHYSIOLOGY
As the ECCO2R system lowers the PaCO2, the alveolar concentration of O2 will increase in accordance with the alveolar gas equation.
By removing CO2, ECCO2R allows ventilation strategies that are focused on oxygenation rather than CO2 elimination. The previously mentioned study by Muller et al.[6] looked specifically at the O2 and CO2 transfer that occurred through the Novalung iLA (AV-ECCO2R) in 96 patients with ARDS. Blood samples were taken before and after the AV-ECCO2R device to calculate the O2 and CO2 content of blood at these points. The flow of blood through the device was also measured and hence the rate of gas transfer could be calculated using Fick’s principle. The transfer capacity for O2 averaged 41.7 ± 20.8 mL/min and for CO2 was 148.0 ± 63.4 mL/min [Figures 2 and 3].
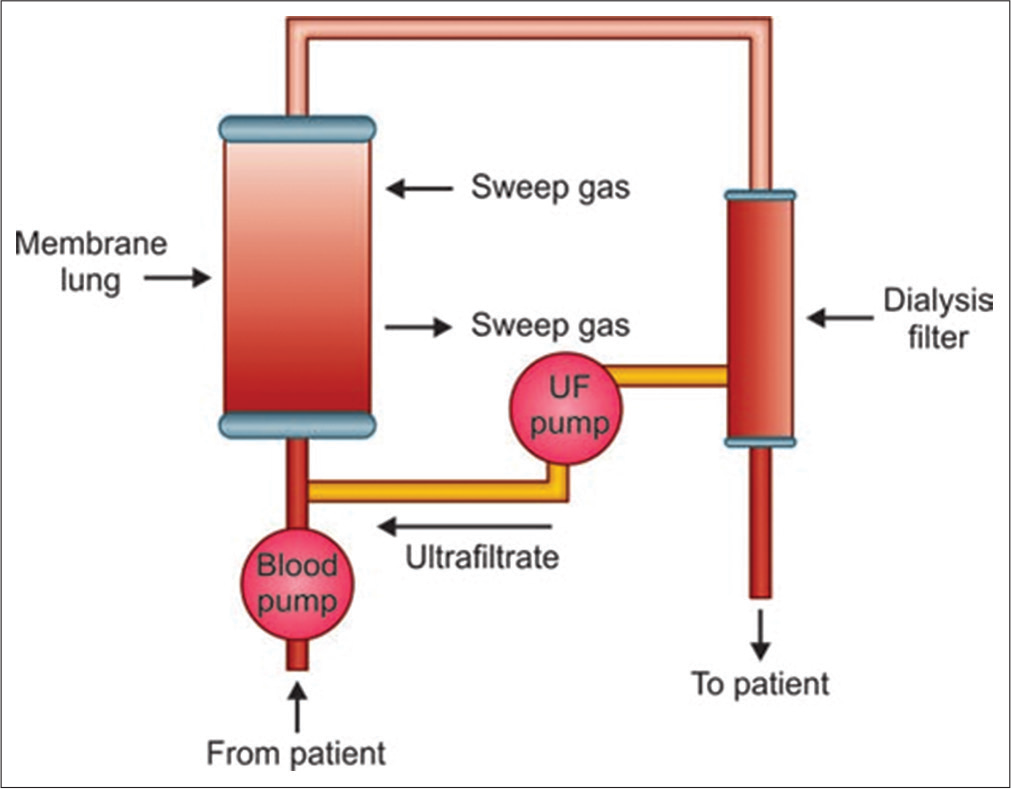
- iLA Activve; circuit diagram.
- Blood is pumped through a membrane lung with a dialysis filter.
RATIONALE BEHIND THE USE OF ECCO2R
Until recently, the primary use of ECCO2R has been as a bridge to recovery in cases of severe hypercapnic acidosis (HCA) that are refractory to mechanical ventilation. In the vast majority of cases, this has been in the context of ARDS, although it has also been used in a variety of other situations. The threshold at which a HCA requires treatment is debatable and will vary depending on the clinical situation but most would agree that there comes a point at which intervention is required.
COMPLICATIONS ASSOCIATED WITH ECCO2R
The complications of AV-ECCO2R and VV-ECCO2R should be looked at separately since the two configurations have different side effect profiles. The most concerning complications of AVECCO2R have been related to arterial cannulation with three reports of limb ischemia requiring amputation in the early literature. Improvements in the cannulae allowed the use of shorter (9 cm vs. 14 cm) and thinner (13 vs. 15 Fr) cannulae for arterial cannulation which with the use of ultrasound to ensure that the internal diameter of the artery is of adequate size (1.5 times the external diameter of the cannula) have reduced complication rates. Hence, the complication rates in [Table 3] are from the most recent prospective study of AV-ECCO2R.[13]
| Complications | Complications rate with AV-ECCO2R |
Complications rate with VV-ECCO2R |
|---|---|---|
| Limb ischemia | 5.9% (3/51) | 0 |
| Compartment syndrome | 1.9% (1/51) | 0 |
| Bleeding during cannulation | 1.9% (1/51) | 0 |
| Cannula thrombosis | 1.9% (1/51) | 16.7% (3/18) |
| Thrombosis of exchange membrane | Not reported | 16.7% (3/18) |
| Pump malfunction | 0 | 5.6% (1/18) |
VV-ECCO2R: Venovenous-Extracorporeal carbon dioxide removal, AV-ECCO2R: Arteriovenous-Extracorporeal carbon dioxide removal
The complication rates for VV-ECCO2R in this table are compiled from the 18 cases of its use that are reported in the literature.[9-12,14] Plasma leakage, heparin-induced thrombocytopenia,[15] hemolysis are some dreaded complications observed all throughout this process.[16] There has also been a report of critical hypotension when AVECCO2R was initiated in a patient who had severe hypoxia and septic shock are other complications.[17]
DISCUSSION
Worku et al. in recent times post-COVID-19 have discussed that driving pressure below conventionally accepted limits of ultra-protective targets can further improve patient centered outcomes compared with standard low-volume and low-pressure ventilation strategies.[18] Ventilator parameters under ECCO2R largely conformed with ultra-protective ventilation targets from a mechanical perspective (driving and plateau pressures).[19] Plateau pressure was ≥25 cm H2O in all 10 cohorts at the outset and was reduced significantly at 24 h with reported values ≤25 cm H2O in seven studies. Driving pressure was >14 cm H2O in just two of the seven studies reporting ∆P at baseline. While it was reduced to <10 cm H2O in five studies, and below 14 cm H2O in all reporting studies, TV remained slightly above 4 mL/kg in the majority.[20-25]
ECCO2R is rightly referred to as a low-flow ECMO.[26] ECCO2R was originally developed to reduce the intensity of mechanical ventilation in patients with acute hypercarbic respiratory failure. There remains an unmet need to better quantify lung injury and to identify patients who may benefit most from a reduction in the intensity of mechanical ventilation. Current evidences do not exclude the possibility to smaller beneficial effects to a subset of patients. At present, as per most recent reviews and studies, ECCO2R should not be used outside of a research clinical trial or only in highly selected cases.[27]
CONCLUSION
VV ECCO2R supported significant reductions in driving pressure at 24 h in moderate-to-severe ARDS, with an overall mortality of 41.6%. While early ECCO2R may facilitate ultra-protective ventilation and mitigate ARDS progression, the benefits are currently offset by the invasiveness of therapy, and limited CO2 removal made possible at low blood flow rates. Significant reductions in respiratory rate may emerge as the key sub-component of mechanical power; hence, reductions in ergotrauma may not be feasible when using very low blood flow rates, potentially limiting the utility of ECCO2R in patients with more severe forms of ARDS, or right ventricular dysfunction. Enrichment of study populations, and reporting data consistently to minimum standards, is critical to meaningfully researching benefits of ECCO2R therapy in ARDS. From renal to pulmonary dialysis, new techniques rise to assist clinicians in the management of critical patients with organ failures. Coupling mild extracorporeal support devices with ultra-protective ventilation represent the most promising possibility to obtain the best therapeutic goals in the severe ARDS patients’ treatment. New strategies for ventilation support are currently under investigation, specifically for the treatment of COPD exacerbations and as a bridge for lung transplant.
Declaration of patient consent
Patient’s consent not required as their identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Arteriovenous extracorporeal membrane carbon dioxide removal In: N1452. London: National Institute for Health and Clinical Excellence; 2008.
- [Google Scholar]
- Control breathing using an extracorporeal membrane lung. Anesthesia. 1977;46:138-41.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Resp Crit Care Med. 1994;149:295-305.
- [CrossRef] [PubMed] [Google Scholar]
- Low-frequency positive pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA. 1986;256:881-6.
- [CrossRef] [Google Scholar]
- Total extracorporeal arteriovenous carbon dioxide removal in acute respiratory failure: A phase I clinical study. Intensive Care Med. 2001;27:1340-51.
- [CrossRef] [PubMed] [Google Scholar]
- Extracorporeal pumpless interventional lung assist in clinical practice: Determinants of efficacy. Eur Resp J. 2009;33:551-8.
- [CrossRef] [PubMed] [Google Scholar]
- Extracorporeal carbon dioxide removal using the Novalung in a patient with intracranial bleeding. Anesthesia. 2007;62:72-4.
- [CrossRef] [PubMed] [Google Scholar]
- Pre-emptive Novalung-assisted carbon dioxide removal in a patient with chest, head and abdominal injury. Anesthesia. 2008;63:767-70.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of a lowflow veno-venous carbon dioxide removal device: Results of an experimental study in adult sheep. Crit Care. 2006;10:R151.
- [CrossRef] [PubMed] [Google Scholar]
- Tidal volume lower than 6 mL/kg enhances lung protection role of extracorporeal carbon dioxide removal. Anesthesiol. 2009;111:826-35.
- [CrossRef] [PubMed] [Google Scholar]
- The use of CO2 removal devices in patients awaiting lung transplant: An initial experience. Transplant Proc. 2010;42:1255-8.
- [CrossRef] [PubMed] [Google Scholar]
- Veno-venous carbon dioxide removal in chronic obstructive pulmonary disease: Experience in one patient. ASIAO J. 2009;55:420-2.
- [CrossRef] [PubMed] [Google Scholar]
- Pumpless extracorporeal interventional lung assist in patients with acute respiratory distress syndrome: A prospective pilot study. Crit Care. 2009;13:R10.
- [CrossRef] [PubMed] [Google Scholar]
- Extracorporeal removal CO2 using a venovenous, low-flow system (Decapsmart) in a lung transplanted patient: A case report. Transplant Proc. 2009;41:1412-4.
- [CrossRef] [PubMed] [Google Scholar]
- Combination of high frequency oscillatory ventilation and interventional lung assist in severe acute respiratory distress syndrome. J Crit Care. 2010;25:436-44.
- [CrossRef] [PubMed] [Google Scholar]
- A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia. Crit Care Med. 2006;34:1372-7.
- [CrossRef] [PubMed] [Google Scholar]
- Bridge to lung transplantation with the novel pumpless interventional lung assist device NovaLung. J Thor Cardiovasc Surg. 2006;131:719-23.
- [CrossRef] [PubMed] [Google Scholar]
- Venovenous extracorporeal CO2 removal to support ultraprotective ventilation in moderate-severe acute respiratory distress syndrome: A systematic review and meta-analysis of the literature. Perfusion. 2022;2:2676591221096225.
- [CrossRef] [PubMed] [Google Scholar]
- ECCO2R therapy in the ICU: Consensus of a European round table meeting. Crit Care. 2020;24:490.
- [CrossRef] [PubMed] [Google Scholar]
- What links ventilator driving pressure with survival in the acute respiratory distress syndrome? A computational study. Respir Res. 2019;20:29.
- [CrossRef] [PubMed] [Google Scholar]
- Ultraprotective ventilation reduces biotrauma in patients on venovenous extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Crit Care Med. 2019;47:1505-12.
- [CrossRef] [PubMed] [Google Scholar]
- Severe hypoxemia: Which strategy to choose. Crit Care. 2016;20:132.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of lowering tidal volume on mortality in ARDS varies with respiratory system elastance. Am J Respir Crit Care Med. 2021;203:1378-85.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory drive in the acute respiratory distress syndrome: Pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020;46:606-18.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory drive in critically Ill Patients. Pathophysiology and clinical implications. Am J Respir Crit Care Med. 2020;201:20-32.
- [CrossRef] [PubMed] [Google Scholar]
- Low flow venoarterial ECMO support management in postcardiac surgery patient. J Card Crit Care TSS. 2021;5:103-7.
- [CrossRef] [Google Scholar]
- Extracorporeal carbon dioxide removal for acute hypercapnic respiratory failure. Ann Intensive Care. 2019;9:79.
- [CrossRef] [PubMed] [Google Scholar]






