Translate this page into:
Evidence-based Medicine: A Narrative Review on the Evolving Opportunities and Challenges
*Corresponding author: Rohan Magoon, Assistant Professor, Department of Anaesthesia, Atal Bihari Vajpayee Institute of Medical Sciences (ABVIMS) and Dr. Ram Manohar Lohia Hospital, Baba Kharak Singh Marg, New Delhi, India.rohanmagoon21@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Agrawal S, Magoon R, Choudhary N, Suresh V, Kumar A, Nagpal VK, et al. Evidence-based Medicine: A Narrative Review on the Evolving Opportunities and Challenges. J Card Crit Care TSS. 2024;8:122-8. doi: 10.25259/JCCC_51_2023
Abstract
Evidence-based medicine (EBM) undeniably classifies as a pre-eminent advance in the clinical approach to decision-making. Although EBM as a topic has been discussed at length, it is more about the process of integrating EBM into practice, wherein the actual debate becomes even more interesting with unique roadblocks cropping up at the very end of the translational highway. Meanwhile, the core concept of EBM has stood firm over decades; it is likely the research landscape and the corresponding intricacies continue to evolve at a rather rampant pace. Evidence-based practice is thus best elaborated in close conjunction with the recent advent of precision medicine, the impact of the coronavirus disease 2019 pandemic, and the ever-compounding present-age research concerns. In this reference, the randomized controlled trials and now the meta-analysis (second-order analysis of analyses) are also being increasingly scrutinized for the contextual veracities and how the quality of the former can be rendered more robust to strengthen our epic pyramid of EBM. Withstanding, the index narrative article is a modern-day take on EBM keeping abreast of the evolving opportunities and challenges, with the noble objective of deliberating a standpoint that aims to potentially bridge some of the existing gaps in the translation of research to patient care and outcome improvement, at large.
Keywords
Coronavirus disease 2019
Evidence-based medicine
Meta-analysis
Precision medicine
Randomized controlled trials
Systematic reviews
Research
“A false conclusion, once arrived at and widely accepted, is not easily dislodged, and the less it is understood, the more tenaciously it is held. --- Georg Cantor, (1845–1918)”
We begin the narrative review on evidence-based medicine (EBM) with this famous saying of a great German mathematician. Drawing motivation from the same and not having ourselves preoccupied with the quantitative side of EBM to demonstrate a white-and-black approach, we concertedly need to work toward an improved understanding of our research ecosystem. The latter, indeed, is becoming way too complex to underline that the present-day task at hand is no longer just about seeing “what no one has seen” but about thinking “what no one has yet thought” regarding that “which is seen to all,” to be able to candidly embrace EBM into our patient care.
BACKGROUND
Over the decades, how we approach medicine has come a long way, evolving from authority, to experience, to EBM.[1,2] tHis transition can well be represented by the progression from “ipse dixit” (Latin), a modus operandi driven by dogmatic expression of opinion, to the practice increasingly moving toward inclusion of the knowledge gained from experience to the traditional teachings.[2,3] Thereby, remarkable improvement in the field of medicine was witnessed as a result of self-reflection and internal audits, nonetheless lacking the requisite standardization with the practice being intricately linked to the skills of the practitioner.[1,2]
Meanwhile, the initial marks of evidence-based practice can be traced to the mid-19th century; it was only in 1996 when the late Prof Sackett et al. formally defined EBM as “the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients.”[1] In reference to the modern-day context, however, where a section of fraternity is engrossed in debating the implications of the evidence-based guideline protocols on physician autonomy,[4] the conduct of EBM has recently been faced with other peculiar challenges that mandate comprehensive elaboration when staging a discussion in the relevant subject.
THE CANONICAL EBM PYRAMID OF PRIORITIES
EBM is envisaged as a hierarchical system that motivates us to seek the best available evidence and guides when classified in accordance with their weightage and subsequently fall back on the evidence in the absence of the higher [Figure 1]. [5] However, it provides a particularly useful teaching tool connoting the cornerstone of the EBM hierarchy. The former pictorial illustration, better known as the EBM pyramid, characteristically demands a methodical literature search and critical appraisal, thereby integrating the best-available evidence with practice, concurrently acknowledging the patient characteristics, and comprehending the context appropriateness of the evidence at hand.[5-8] Looking closely through this pyramid, two important practical aspects emerge – the first one refers to the source of evidence that EBM practitioners can use, as epitomized by the 6S version of the pyramid, representing a hierarchy spanning from (S)tudies, (S)ynopses, (S)ynthesis, (S)ynopses of synthesis, and (S)ummaries to (S)ystems;[9] whereas the second aspect pertains to the questions surrounding the traditional pyramid being over simplistic, at times. In the early 2000s, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group devised a framework where the certainty of evidence was premised on a multitude of factors and not solely dictated by the study design.[10] Meanwhile, the former framework by itself besets the conventional pyramid of priorities, a riveting perspective by Subbiah states that the EBM pyramid in its current form is merely the tip of the iceberg, offering shallow evidence for the care of a generic patient.[6] They further emphasize the need for a deep synthesis and amalgamation of the natural history data to achieve the “next-gen(eration) EBM.” Herein, they anticipate that exploring the potential of multidimensional evidence procreation would be challenged by the extraction-collation mining of large datasets in addition to the genomics and other “omics” analysis (the discussion of the role of which follows) not without highlighting the lucrative opportunities in acquiring data offered by the innovations in wearables, sensor technology and the Internet of Medical tHings (IoMT) Architecture.[6,8]
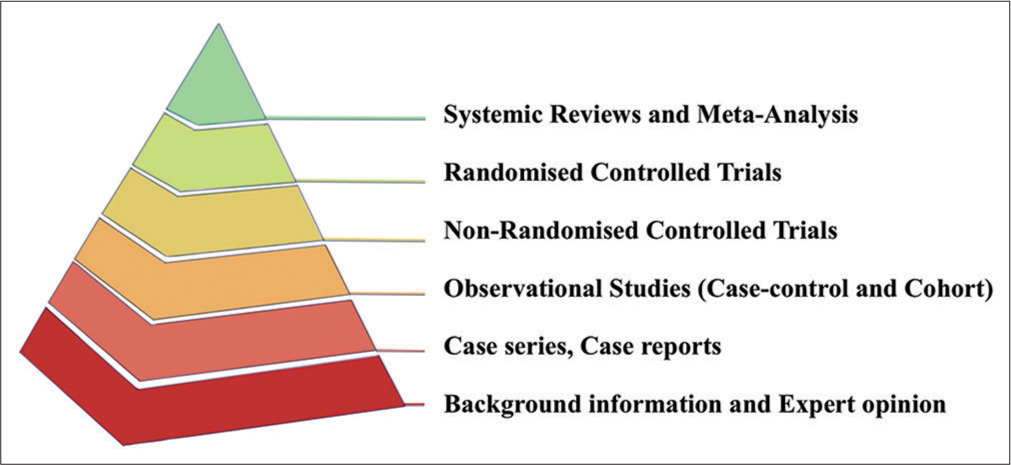
- The basic construct of the evidence-based medicine pyramid, the tiers cemented by “critical appraisal.”
EBM, IN THE ERA OF PRECISION MEDICINE (PM)
Of late, the undertone of PM in practice has steadily been rising to the forefront across all branches of medicine.[4,8,11,12] PM tailors the diagnosis and treatment of diseases to the individual attributes premised on diverse facets of patient-centric characteristics, as outlined in [Table 1].[11,12] Propounding the notion of administering the right treatment to the right patient at the right time, practice is transiting from a kitchen sink to a personalized approach, maximizing the value of specialized services in anesthesia and critical care.[11-13] With the revolutionary trend of PM, translational research and novel domains like the “omics” [as outlined in Table 1] are actively transcending into perioperative medicine to feature as “Anesthesiomics.”[11,12,14]
|
Albeit gaining wide recognition in disease prevention treatment, the scope of precision perioperative medicine remains far from fully explored. To name a few; hemodynamic optimization, circulatory assistance, anesthesia-critical care pharmacological regimens, coagulation-bleeding management, blood and blood-product transfusion, mechanical ventilation, analgesic therapy, prediction of end-organ complications, and contextual protective strategies serve as pertinent contextual examples for the application of PM in perioperative medicine.[8,11] As an exemplar, Shah et al. categorize the post-cardiac surgery cohort with vasoplegia as poor responders, responders, sustainers, and rebounders while studying their response to hydroxocobalamin.[15] With more such research experiences accruing in the literature on the role of patient-centered care (PCC), PM is captivating enhanced attention in anesthesia and critical care.[16]
Simultaneously, an argument regarding the programs, guidelines, and protocols being the antithesis of PM has gained recent impetus, as elucidated in an editorial by Columb and Hopkins.[17] Guidelines can be peculiarly rigid in nature, targeted to an average patient for the mentioned situation; nonetheless, every patient is unique and may or may not fall in that average. tHus, much against the “one-size-fits-all” regime, proponents of PM propose that such standardized therapies fail to cater to the widely prevalent heterogeneity in patient characteristics and the patient-related response to therapy in practice.[8,11] To that end, another interesting editorial on the implications of practice variability by Sessler, questions the basis for assigning a free pass to clinical pathways, buttressing the need for robust context-sensitive evidence.[18]
That being said, sackett et al., during their very first writing on ebm, emphasized that it is all about integrating individual clinical expertise with the best external evidence.[1] therefore, it becomes equally worthwhile to appreciate that EBM is not a “cookbook” medicine and a personalized approach can well be appreciated within its’ realms, and apart from conceptualizing PM as a paradigm shift and seeking the dichotomy (if at all, there exists one), a middle ground can be chosen to harmonize PM and EBM as “evidence-based precision medicine” with the noble objective of improving the patient outcomes, at large.[1,4,19]
THE GLOBAL PANDEMIC, NOT WITHOUT ITS’ DISTINCT IMPACT ON EBM
The coronavirus disease 2019 (COVID-19) potentially challenged the EBM in principle, which had been taught to us for long. The pandemic had thrown into relief the pivotal differences between the evidence-based practice and the traditional form of care that lacked a strong evidentiary background, likely owing to a considerably perturbed demand-supply equilibrium of the scientific evidence required to battle the unfamiliar viral enemy.[20,21]
The fundamentals of EBM, i.e., curtailing the use of non-documentary knowledge for clinical decision-making, stood defeated during the pandemic. Journal houses were necessitated to furnish early publications, having had to hasten their peer review, which further heightened the prevailing dilemma regarding the requisite evidence for clinical decision-making in COVID-19. To add to it, considering the hyperdynamic and volatile nature of the existing research, the pandemic spawned too many uninformative or, at times, misleading clinical trials and reviews, which were again detrimental to the overall quality of the available evidence.[21,22] As an exemplar, the age-old, well-reputed journal, “The Lancet” had to retract an online published research article on chloroquine therapy for COVID-19 due to concerns raised with respect to the veracity of the research data, with the Journal lamenting having collaborated to contribute in good faith, during the unprecedented times of great need presented by the global viral pandemic.[23]
Talking further on the massive increase in research across the board during the pandemic, “publication hyperinflation” is another epiphenomenon that deserves mention. Tsutsumi et al. Recently evaluated the publications in intensive care medicine in a meta-epidemiological study spanning more than three decades.[24] they outlined a hyperinflation in the number of randomized controlled trials (rcts), increasing from 66 in 1990 to 309 in 2021, which equated to as much as 26 rcts per month. Of note, their analysis also delineated the number of systematic reviews (srs) to be escalating at an even higher pace – which from 2 in 1990, increased to 327 in 2016 and reached 519 in 2021, implying the ratio of srs/rcts >1, i.e., 1.68 srs for every RCT in 2021 (519/309, as elucidated above).[24] this heralds the growing wave of srs and meta-analysis (mas), which might/might not be reasonable but certainly no substitutes for well-conducted multicentric large rcts, which would be subsequently discussed in the article.[25]
Specific to the pandemic though, the media and the political interference had their own peculiar impact, which can simultaneously not be undermined. Anecdotal shreds of evidence were being circulated on social media, propagating a “viral infodemic” which was by no means an easy battle for the scientific community. The pandemic having encouraged preprints, the researchers posted their findings online, which, albeit lacking external scrutiny, were widely shared on social media and disseminated at an even accelerated pace in the current age of liking and retweeting.[21,22]
Meanwhile, some may argue that medical practice is often dogged by dogma in several practical areas of management; the COVID-19 pandemic, in addition, might have exposed some of the pre-existing shortcomings of our practice of EBM.[20-22,26] However, on a more positive note, there is always light at the end of the tunnel and the silver lining is indeed offered by the valuable research lessons learned from the pandemic, particularly on how to balance the hope and the hype when reeling under a perpetual scanner.
MAINTAINING “TOP-NOTCH” STANDARDS AT THE “TOP TIER” OF EBM
Ever since the first description of EBM by Sackett et al., EBM has evolved.[1] It is thus imperative to reflect upon the concerns that have evolved alongside as well. EBM (what we aim to practice) frequently presents us with questions and nuances, especially relevant for RCTs and MAs, which feature at the top-tier level of evidence where maintaining top-notch standards is the holy grail.[5] tHis segment is discussed under the subheadings covering the specific research concerns to some generalized issues pertinent to the present times, to finally summarize the elements limiting confidence in medical research.
Apprising the specific research concerns
RCTs are a one-of-a-kind scientific experiment wherein a random allocation of the participants among the compared treatments achieves a sufficient degree of control over the confounding factors to enable a clinically useful comparison of the therapies under evaluation, provided it features a satisfactory study design and is meticulously conducted with context-appropriate blinding while enrolling an adequate sample size. As Goodman aptly puts it: “Statistics, which are at the heart of EBM, are not even proof; they are only probabilities,” The estimates of probability mandate closer attention.[27] For instance, given the long-standing misinterpretations of the P-value, the American Statistical Association issued a statement on the context, process, and purpose of the P-values.[28] Moreover, the use of P-value thresholds as evidence of treatment effects in RCTs fails to cater to the number of participants or, for that matter, events in the trial. To explain it lucidly, for a trial depicting a low event rate, a relatively small change in the number of events either in the treatment or the control group may alter the outcome from being statistically significant to non-significant (P ≥ 0.05).[29] Hence, The P-value serves as a poor representative of the fragility of the involved statistical analysis. A recent SR by Demarquette et al. outlines RCTs to be increasingly fragile, considering their discovery of a median “fragility index (FI)” of 4 {1–8} in a total of 65 statistically positive trials with only about 10% of the RCTs manifesting a FI in excess of 10 (FI, representing the minimum number of participants whose status would have to change from “non-event” to “event,” to render the statistically significant results non-significant while assessing two-group comparisons on binary criteria).[30] Amidst a heightened number of investigations frequently outlining a low FI (<5) across assorted medical literature, another large specific analysis of RCTs in pediatric anesthesiology also revealed a low median FI value of 3 {1–7} while evaluating a total of 172 trials over 25 years, to raise valid concerns regarding the robustness of the existing RCTs.[29]
In addition, issues surrounding scientific transparency make for an equally important debate. Of note, Carlisle, in a probe of RCTs published over three years in a leading anesthesia journal, scrutinized 153 trials having accessed their individual-level data to elucidate that the analysis of the individual-level data information increases the odds of a trial being labeled “false” or “zombie” (if, fatally flawed with credibility) by a considerable 47-fold and 79-fold, respectively.[31] Enhanced data availability herein contributed significantly to the detection of errors in these trials when compared to the trials lacking individual patient data (44% vs. 2% for false trials and 26% vs. 1% for zombie trials).[31]
MA, albeit positioned at the top tier in the EBM pyramid, is not free from concerns. Considering the highest-level recommendations are premised on MAs, assessing the evidence they offer becomes even more important. Drawing nomenclature from the Greek prefix, “meta” denotes “after or transcending,” where it needs to be borne in mind that MA essentially classifies as a second-order analysis of analyses, subject to the influence of the quantity–quality nature of the component studies and the concurrent potential biases, often the publication bias.[32-34] The same is exemplified by the existence of MAs with contradictory results on a given research topic despite strictly adhering to the formal Preferred Reporting Items for SRs and Meta-Analysis (PRISMA) guidelines.[35] Ahead of the discussion on the role of appropriate antecedent literature review and the inclusion of gray literature in the subject alongside the larger implications of heterogeneity while conducting a second-order analysis,[32,36,37] there appears to be a conspicuously growing wave of SRMAs given the ready availability of the requisite software.[25] Duly accepting the value of tools like trial sequential analysis in aiding rigorous assessment of the cumulative evidence by featuring sequential monitoring boundaries while estimating the required information size,[38] the enormous rise in the number of MAs might not be justified as discussed in the previous section on publication hyperinflation.[24,25] With more moot SRMAs being added to our research ecosystem, sustaining clinical controversies (which, in certain cases, might not actually exist any longer) is a worrying trend, deeming it imperative for us to introspect whether we are getting the lemonade from the lemons or not?[25]
Other general issues of the modern-day
The phenomenon of “spin” in the present-day rcts (characterized by the manipulation of language to mislead the readers from the likely truth of the research results), clearly speaks of the rhetorical techniques being employed in conveying medical science.[30,39] In an analysis of 162 articles published in high-impact anesthesia journals, Demarquette et al. Discovered that as much as 40% of the statistically negative trials potentially misled the readers through the inclusion of “spin”ning tricks.[30] Such reporting strategies to suggest a benefit of an experimental intervention despite a statistically non-significant difference in the primary outcome is an evolving plague to scientific research. Concerted efforts must be directed toward the detection and reduction of spin in rcts to safeguard our “research-evidence translational continuum.”[39]
At the same time, the researchers need to be particularly mindful of employing the retracted literature base while formulating their research projects and/or drafting the manuscripts for publication. Herein, citation of retracted literature is an additional menace, with the problem compounding further when the former has methodological implications on the primary investigation.[40] Even in the second-order analysis of analyses, retracted literature can significantly distort the findings of the top-tier evidence.[41]
Furthermore, any discussion on EBM in today’s age is incomplete without deliberating on the intriguing influences of artificial intelligence (AI) on scientific research. [Figure 2] illustrates the pros and cons of AI in research and analysis, leaving the question open to debate whether open AI is a boon or a bane to the scientific community.[6,42-44]
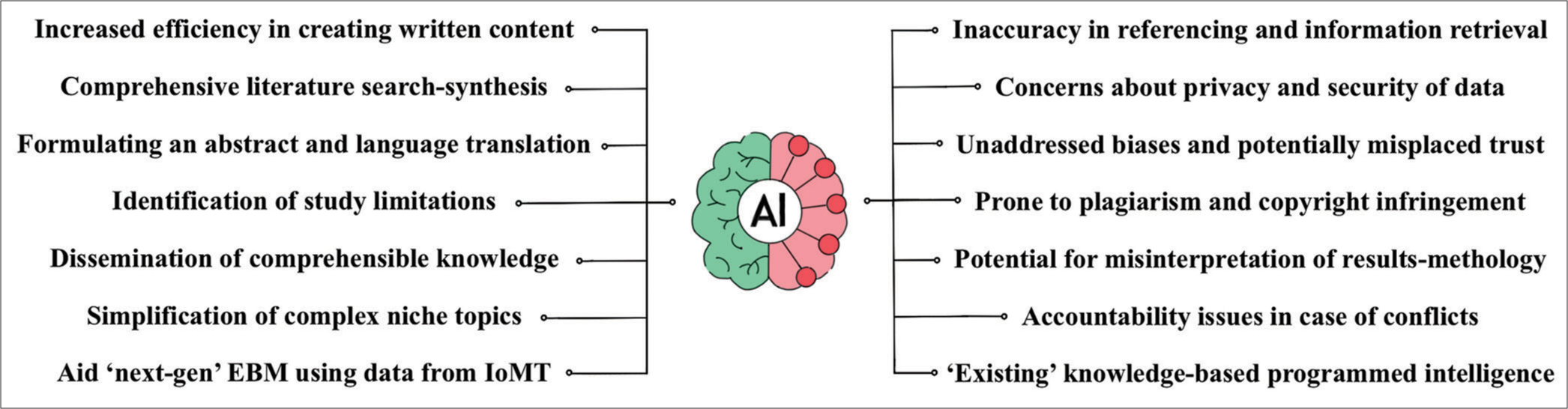
- The pros and cons of artificial intelligence in research and analysis.
Moore et al. 3Fs limiting the confidence in research
As a useful guide to comprehending the features which can limit confidence in the contemporary literature, Moore et al., alongside narrating the multitude of relevant factors, present a context-appropriate classification of the same into the 3Fs, eventually culminating as either (F)lawed, (F)utile, or (F) abricated research [Table 2].[45]
|
FUTURE DIRECTIONS
The quintessential altercation in the subject regarding the peak of the pyramid continues to intensify with some arguing in favor of well-conducted RCTs mimicking real-world situations better than any other study design, thus minimizing the likelihood of confounding. The usual counterargument is that the best evidence is provided by SRMAs, given the integration of all the relevant evidence, purportedly offering more reliable answers than a well-conducted single study, often mistaking an augmented external validity achieved by pooling of data, which, on the contrary, presents a sizeable risk of systematic error.[32,46] Beyond this debate, the future is rather about refining our evidence to include patient-reported outcome and experience measures, shared decision-making, and seeking viable solutions to cultivate an increasing trust in the anesthesiologists’ endeavors in providing PCC while practicing the best-available evidence.[16,47] However, the best evidence at hand is unlikely to always follow the hierarchy propounded by the EBM pyramid. Notably, dependent researchers put forth the need for upending the epic pyramid for certain clinical situations.[48] To that end, it is hoped that the future awaits a better pyramid, given there exist no shortcuts to the truth – whatever the clinical evidence looks like and wherever it features on this pyramid.[46] We believe the “3Es” would be instrumental in achieving this pinnacle, i.e., adequately backed by robust (E)vidence, physician (E)xperience, and meeting the patient (E)xpectations.
CONCLUSION
EBM is no less than a dynamic goal post wherein every opportunity be avidly sought to build a thorough, reproducible, and pragmatic basis for promoting good clinical practice. Acknowledging the complex, multifactorial, and deeply situational nature of our practice, today, there is an equally (if, not more) challenging need to meticulously interpret the ever-growing research repository to figure out what truly classifies as practice-changing evidence. Meanwhile, it remains to be seen in the future how PCC integrates with EBM in the upcoming age of AI; it would only be opportune to emphasize that our “param kartavya” of “primum non nocere” should unfailingly be observed, throughout the not so predictable evolving process.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Evidence Based Medicine: What It Is and What It Isn't. BMJ. 1996;312:71-2.
- [CrossRef] [PubMed] [Google Scholar]
- The Emperor's New Clothes: A Critical Appraisal of Evidence-based Medicine. Int J Med Sci. 2018;15:1397-405.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacotherapy in Allergy Medicine: From 'Ipse Dixit' to the Evidence-based Medicine. Curr Opin Allergy Clin Immunol. 2020;20:407-13.
- [CrossRef] [PubMed] [Google Scholar]
- From Precision of the Evidence to the Evidence for Precision: An Intriguing Odyssey! J Anaesthesiol Clin Pharmacol. 2022;38:153-4.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence-based Medicine: Revisiting the Pyramid of Priorities. J Bodyw Mov Ther. 2012;16:42-9.
- [CrossRef] [PubMed] [Google Scholar]
- The Next Generation of Evidence-based Medicine. Nat Med. 2023;29:49-58.
- [CrossRef] [PubMed] [Google Scholar]
- Validity of Meta-analysis in Diabetes: Meta-analysis is an Indispensable Tool in Evidence Synthesis. Diabetes Care. 2013;36:3368-73.
- [CrossRef] [PubMed] [Google Scholar]
- Implications of Practice Variability: Comment. Anesthesiology. 2020;133:943-4.
- [CrossRef] [PubMed] [Google Scholar]
- GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ. 2008;336:924-6.
- [CrossRef] [PubMed] [Google Scholar]
- Precision Cardiac Anesthesia: Welcome Aboard! J Cardiothorac Vasc Anesth. 2020;34:2551-2.
- [CrossRef] [PubMed] [Google Scholar]
- Precision Medicine: Hype or Hope? Trends Cardiovasc Med 2022:S1050-1738(22)00139-6.
- [CrossRef] [PubMed] [Google Scholar]
- Postoperative Pain Management: Are We Ready to Move Beyond the 'Kitchen-sink' Approach? Anaesthesia. 2023;78:807-10.
- [CrossRef] [PubMed] [Google Scholar]
- Anesthesiomics: Could a New Name Be Coined for Anesthesia? Anesth Pain Med. 2020;10:e100988.
- [CrossRef] [Google Scholar]
- Hydroxocobalamin for the Treatment of Cardiac Surgery-associated Vasoplegia: A Case Series. Can J Anaesth. 2018;65:560-8.
- [CrossRef] [PubMed] [Google Scholar]
- From Evidence-based Medicine to Patient-centered Care. Korean J Anesthesiol. 2023;76:265-6.
- [CrossRef] [PubMed] [Google Scholar]
- Programmes, Guidelines and Protocols-the Antithesis of Precision Medicine? Br J Anaesth. 2015;115:485-7.
- [CrossRef] [PubMed] [Google Scholar]
- Implications of Practice Variability. Anesthesiology. 2020;132:606-8.
- [CrossRef] [PubMed] [Google Scholar]
- Next Generation Evidence-based Medicine: Individualised, Personalised and Humanised. Int J Rheum Dis. 2013;16:615.
- [CrossRef] [PubMed] [Google Scholar]
- Covid-19: Exposing the Lack of Evidence-based Practice in Medicine. Hastings Cent Rep. 2020;50:77-8.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges for the Practice of Evidence-based Medicine during COVID-19 Pandemic (Practice of Evidence-based Medicine in the New Normal) Indian J Anaesth. 2022;66:290-3.
- [CrossRef] [PubMed] [Google Scholar]
- Compounded Research Challenges amid the COVID-19 Pandemic. Anaesth Crit Care Pain Med. 2020;39:689-90.
- [CrossRef] [PubMed] [Google Scholar]
- Retraction-Hydroxychloroquine or Chloroquine with or without a Macrolide for Treatment of COVID-19: A Multinational Registry Analysis. Lancet. 2020;395:1820.
- [CrossRef] [PubMed] [Google Scholar]
- Publication Hyper-inflation in the Field of Intensive Care. Intensive Care Med. 2023;49:706-7.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analyses of Clinical Trials: Are We Getting Lemonade from Lemons? Br J Anaesth. 2022;128:233-5.
- [CrossRef] [PubMed] [Google Scholar]
- Challenging Management Dogma Where Evidence is Non-Existent, Weak or Outdated. Intensive Care Med. 2022;48:548-58.
- [CrossRef] [PubMed] [Google Scholar]
- Anaesthesia and Evidence-based Medicine. Anaesthesia. 1998;53:353-68.
- [CrossRef] [PubMed] [Google Scholar]
- The ASA's Statement on P-values: Context, Process, and Purpose. Am Stat. 2016;70:129-33.
- [CrossRef] [Google Scholar]
- The Fragility Index of Randomized Controlled Trials in Pediatric Anesthesiology. Can J Anaesth. 2023;70:1449-60.
- [CrossRef] [PubMed] [Google Scholar]
- Spin and Fragility in Randomised Controlled Trials in the Anaesthesia Literature: A Systematic Review. Br J Anaesth. 2023;130:528-35.
- [CrossRef] [PubMed] [Google Scholar]
- False Individual Patient Data and Zombie Randomised Controlled Trials Submitted to Anaesthesia. Anaesthesia. 2021;76:472-9.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis Under the Spotlight: Focused on a Meta-analysis of Ventilator Weaning. Crit Care Med. 2008;36:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis: Convenient Assumptions and Inconvenient Truth. Crit Care Med. 2008;36:328-9.
- [CrossRef] [PubMed] [Google Scholar]
- When Do We Need to do Meta-analysis!!! Indian J Anaesth. 2023;67:673-4.
- [CrossRef] [PubMed] [Google Scholar]
- Contradictory Findings of Two Recent Meta-Analyses: What are We Supposed to Believe About Anesthetic Technique in Patients Undergoing Cardiac Surgery? J Cardiothorac Vasc Anesth. 2021;35:3841-3.
- [CrossRef] [PubMed] [Google Scholar]
- Significance of Including Grey Literature Search in Systematic Reviews and Meta-analyses. Saudi J Anaesth. 2023;17:295-6.
- [CrossRef] [PubMed] [Google Scholar]
- Heterogeneity in Meta-analyses of Treatment of Acute Postoperative Pain: A Review. Br J Anaesth. 2013;111:897-906.
- [CrossRef] [PubMed] [Google Scholar]
- Trial Sequential Analysis: Adding a New Dimension to Meta-analysis. Anaesthesia. 2020;75:15-20.
- [CrossRef] [PubMed] [Google Scholar]
- Safeguarding Anaesthesia Research from Spin. Br J Anaesth. 2020;125:e460-2.
- [CrossRef] [PubMed] [Google Scholar]
- Context Analysis of Continued Citation of Retracted Manuscripts Published in Anesthesiology Journals. Anesth Analg. 2022;135:1011-20.
- [CrossRef] [PubMed] [Google Scholar]
- On the Road to Make KJA's Review Process Robust, Transparent, and Credible: Retracted Study in Systematic Review. Korean J Anesthesiol. 2022;75:197-9.
- [CrossRef] [PubMed] [Google Scholar]
- ChatGPT and Conversational Artificial Intelligence: Friend, Foe, or Future of Research? Am J Emerg Med. 2023;70:81-3.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding Basic Principles of Artificial Intelligence: A Practical Guide for Intensivists. Acta Biomed. 2022;93:e2022297.
- [Google Scholar]
- Exploring Artificial Intelligence in Anesthesia: A Primer on Ethics, and Clinical Applications. Surgeries. 2023;4:264-74.
- [CrossRef] [Google Scholar]
- Flawed, Futile, and Fabricated-features that Limit Confidence in Clinical Research in Pain and Anaesthesia: A Narrative Review. Br J Anaesth. 2023;130:287-95.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis as Evidence: Building a Better Pyramid. JAMA. 2014;312:603-5.
- [CrossRef] [PubMed] [Google Scholar]
- Patient-reported Outcome Measures and Patient-reported Experience Measures. BJA Educ. 2017;17:137-44.
- [CrossRef] [Google Scholar]
- Evidence-based Medicine: Time to Upend the Pyramid for Some Clinical Situations? Br J Anaesth. 2018;120:1134-5.
- [CrossRef] [PubMed] [Google Scholar]







