Translate this page into:
Blood Clot Consensus Recommendations on Bleeding Management during Cardiac Surgery in Low-Resource Settings using E-Delphi Methodology
*Corresponding author: Poonam Malhotra Kapoor, Department of Cardiac Anaesthesia and Critical Care, Cardiothoracic and Neurosciences Centre, All India Institute of Medical Sciences, New Delhi, India. drpoonamaiims@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kapoor PM, Kanchi M, Shastri N, Genny SK, Jacob SI, Tailor KB, et al. Blood Clot Consensus Recommendations on Bleeding Management during Cardiac Surgery in Low-Resource Settings using E-Delphi Methodology. J Card Crit Care TSS. 2025;9:9-20. doi: 10.25259/JCCC_49_2024
Abstract
India conducts around 240,000 adult cardiac surgeries annually, with bleeding and transfusions being common complications that can worsen outcomes. Implementing patient blood management strategies can reduce unnecessary transfusions and improve results. With emerging management options and point-of-care testing, the need for standardized bleeding management during cardiac surgery in India became evident. The Blood Clot (Indian Bleeding Management during Cardiac Surgery) Working Group convened 3 times (one in-person, two virtual) to discuss and vote on consensus-based recommendation statements derived from a Delphi process. The online Delphi platform enabled anonymous voting, providing real-time statistical insights during discussions. Using the accurate consensus reporting document methodology, 26 recommendations were finalized, covering pre-, intra-, and post-operative bleeding management. The recommendations included both Thrombelastography (TEG)/Rotational thromboelastometry (ROTEM) and non-TEG/ROTEM-based algorithms, along with specific guidance for managing bleeding in cyanotic congenital heart disease surgery. These consensus-based recommendations represent the first comprehensive, India-specific guidelines for managing bleeding during cardiac surgery, aiming to optimize practices and potentially set a new standard of care. This approach could also influence global practices in similar contexts.
Keywords
Bleeding
Cardiac surgery
Point-of-care testing
Patient blood management
Indian recommendations
Delphi
INTRODUCTION
Every year in India, around 240,000 adult cardiac surgeries, including valve surgeries and coronary artery bypass grafting, are performed in approximately 420 centers.[1]
Bleeding, a significant complication, affects 2–15% of cardiovascular surgeries, leading to high morbidity.[2-5] Causes include platelet dysfunction, clotting factor dilution, hypothermia, and fibrinolysis. Severe bleeding can necessitate re-exploration in 3–14% of cases, with 50–67% of these revealing a surgically correctable source.[6-10] Bleeding and surgical re-exploration are both independent predictors of an adverse outcome in cardiac surgery.[11]
Comprehensive bleeding assessment during surgery is challenging due to the dynamic coagulation cascade.[12,13] A multimodal approach, supported by institutional protocols and point-of-care testing, is vital, especially in India with limited resources. The Indian Bleeding Management during Cardiac Surgery (Blood Clot) Working Group developed 26 recommendation statements based on expert consensus to guide clinical practice. These recommendations, derived from a Delphi process, aim to provide the best treatment paradigm for bleeding management in cardiac surgery, considering local context and practical experiences. Regular reassessment of these recommendations is advised to incorporate new evidence and technologies.
METHODOLOGY
The study protocol was not prospectively registered. Led by Prof. Yatin Mehta and Prof. Poonam Malhotra, the Delphi method leveraged the expertise of nine cardiac anesthesiologists selected for their extensive experience in managing bleeding during cardiac surgery. This need for clinical guidelines specific to India was recognized during an advisory board meeting in June 2023 in New Delhi, covering various adult and pediatric cardiac surgeries. A literature review informed the initial thematic statements, focusing on bleeding management techniques, patient outcomes, blood management strategies, and advances in monitoring and intervention methods. The review included databases such as PubMed, Cochrane Library, Scopus, Web of Science, and Google Scholar from January 2016 to February 2023.
The E-Delphi platform was used for the survey, without a pilot study. Key clinical questions were reviewed by experts, resulting in 26 thematic statements. These statements were discussed in three meetings (one in-person, two virtual), moderated by Prof. Malhotra, with live changes made by a medical writer. Consensus was defined as ≥80% agreement, with statements excluded if they did not achieve a minimum of 50% consensus. All statements reached the consensus threshold in the first round, with results visible in real-time on the E- Delphi portal.
Two initial recommendations were omitted as they represented general practice. Feedback was provided after each meeting, with anonymized votes visible at all times. Participants were reimbursed only for the first meeting. Descriptive statistical analysis was conducted to summarize responses. Ethics Committee approval was not required as the study did not involve patient-specific therapies or data. The methodology adhered to accurate consensus reporting document (ACCORD) guidelines[14] and good data protection practices.
RESULTS
A total of 26 statement recommendations (R1 to R26) were developed. The statements were divided into “General recommendations,” “Pre-operative,” “Intra-operative,” and “Post-operative” recommendations, as follows:
General recommendations
R1. Transfusion practices during cardiac surgery must be “restrictive,” rather than “liberal,” and supported by measures like:
Intensifying treatment of anemia in the pre-operative period,
Use of minimally invasive surgical techniques,
Use of standardized protocols guiding the use of blood components and
Minimizing intra- and post-operative iatrogenic blood loss (77.78% Strongly agree; 22.22% Agree; Standard deviation [SD] = 0.416).
R2. The management options for non-surgical bleeding during cardiac surgery include the use of packed red blood cells (PRBC), cryoprecipitate, platelet concentrate (random donor/single donor), fresh frozen plasma (FFP), fibrinogen concentrate, prothrombin complex concentrate (PCC), and recombinant factor VIIa (87.5% Strongly agree; 12.5% Agree; SD = 0.331).
R3. Drugs such as antifibrinolytics, for example, tranexamic acid, epsilon aminocaproic acid (EACA), and desmopressin may be added in cases of continued bleeding. Protamine dose is about 70% of the primary heparin dose and more than 1005 should strictly be avoided. Antidotes for direct thrombin inhibitors, hemadsorption therapy for Novel Oral Anticoagulants, and their antidotes should be added depending on the individual anticoagulants used (75% Strongly agree; 25% Agree; SD = 0.433).
R4. The recommended point-of-care/viscoelastic tests in a bleeding patient to diagnose/treat/monitor transfusion during cardiac surgery are activated clotting time (ACT), hematocrit, extrinsic thromboelastometry (EXTEM), fibrinogen thromboelastometry (FIBTEM), intrinsic thromboelastometry (INTEM) and heparin thromboelastometry (HEPTEM), Kaolin thrombelastography (TEG), heparinase TEG, rapid TEG and TEG Functional Fibrinogen/Multiplate/Rotational thromboelastometry (ROTEM) platelet/Verify now/TEG platelet mapping and QUANTRA, whichever is available in the institution of a POC testing device (87.5% Strongly agree; 12.5% Agree; SD = 0.331).
Pre-operative recommendations
R5. A pre-operative multidisciplinary team (cardiac anesthesiologists, cardiac surgeon, intensive care unit [ICU] physician, transfusion medicine specialist, clinical pharmacist, perfusionist, and hematologist) to discuss aspects such as invasive monitoring, cannulation sites, management of cardiopulmonary bypass (CPB), post-bypass inotropic support, chest closure, ventilatory support, and blood conservation management planning is encouraged for best alignment (87.5% Strongly agree; 12.5% Agree; SD = 0.331).
R6. Pre-operatively patients requiring and/or are at high probability of transfusion (advanced age, complex re-do surgery) can be identified by checking for iron deficiency anemia, non-iron deficiency anemia, platelet count, and fibrinogen level. Platelet function testing should additionally be done in patients taking purinergic receptor type Y, subtype 12 inhibitors or dual anti-platelet therapy (77.78% Strongly agree; 22.22% Agree; SD = 0.416).
R7. In patients who have pre-operative anemia, or in those who refuse a blood transfusion, it is recommended to administer pre-operative oral iron therapy or erythropoietin-stimulating agents along with oral iron supplementation several days before cardiac surgery to increase red cell mass (88.89% Strongly agree; 11.11% Agree; SD = 0.314).
R8. In patients undergoing elective cardiac surgery, anti-platelet medications such as ticagrelor should be withdrawn preoperatively for a minimum of 3 days, clopidogrel for 5 days, and prasugrel for 7 days, and stop all group IIb/IIIa inhibitors for 4–6 h before surgery. It is reasonable to continue low-dose aspirin therapy until the time of surgery. In patients undergoing emergency cardiac surgery on P2Y12 inhibitors or direct factor Xa inhibitors such as apixaban or rivaroxaban with high bleeding risk, consider hemadsorption (87.5% Strongly agree; 12.5% Agree; SD = 0.331).
R9. For patients on pre-operative warfarin, it should be stopped 3–5 days before surgery, and other non-Vitamin K-dependent oral anticoagulants should be stopped 48 h before surgery and bridged with heparin (87.5% Strongly agree; 12.5% Agree; SD = 0.331).
Intraoperative recommendations
R10. Intraoperative autologous blood donation in adult patients with hemoglobin levels (>13 g/dL) may be considered to reduce post-operative transfusions (88.89% Strongly agree; 11.11% Agree; SD = 0.314).
R11. Intraoperatively, hemostasis should be maintained, and blood loss should be reduced by minimizing hemodilution, maintaining individual heparin and protamine titration, maintaining normothermia and normal pH and preventing fibrinolysis (88.89% Strongly agree; 11.11% Agree; SD = 0.331).
R12. A baseline ACT is estimated. The ACT is maintained at more than 480 s on cardiopulmonary bypass after an intravenous administration of unfractionated heparin (UFH) of 300–400 IU/kg. For off-pump surgery, UFH of 100–200 U/kg with a target ACT of 250–300 s is recommended (77.78% Strongly agree; 22.22% Agree; SD = 0.416).
R13. Intraoperatively, during CPB, the factor reaching critical levels earliest is fibrinogen, which leads to bleeding. Cryoprecipitate and/or fibrinogen concentrates can be supplemented in the post-bypass period to correct hypofibrinogenemia (87.5% Strongly agree; 12.5% Agree; SD = 0.331).
R14. Standardized hemostatic algorithms that incorporate point-of-care testing, such as with viscoelastic devices and with pre-defined intervention triggers should always be preferred to diagnose/treat/monitor transfusion practices during cardiac surgery over anesthesiologists’/cardiac surgeons’ clinical discretion and conventional coagulation assays (25% Strongly agree; 75% Agree; SD = 0.433).
R15. Pre-emptive steps should be taken by a cardiac anesthesiologist (CA) intraoperatively on an individualized patient basis, as shown in Flowchart 1 (44.44% Strongly agree; 44.44% Agree; SD = 1.197).
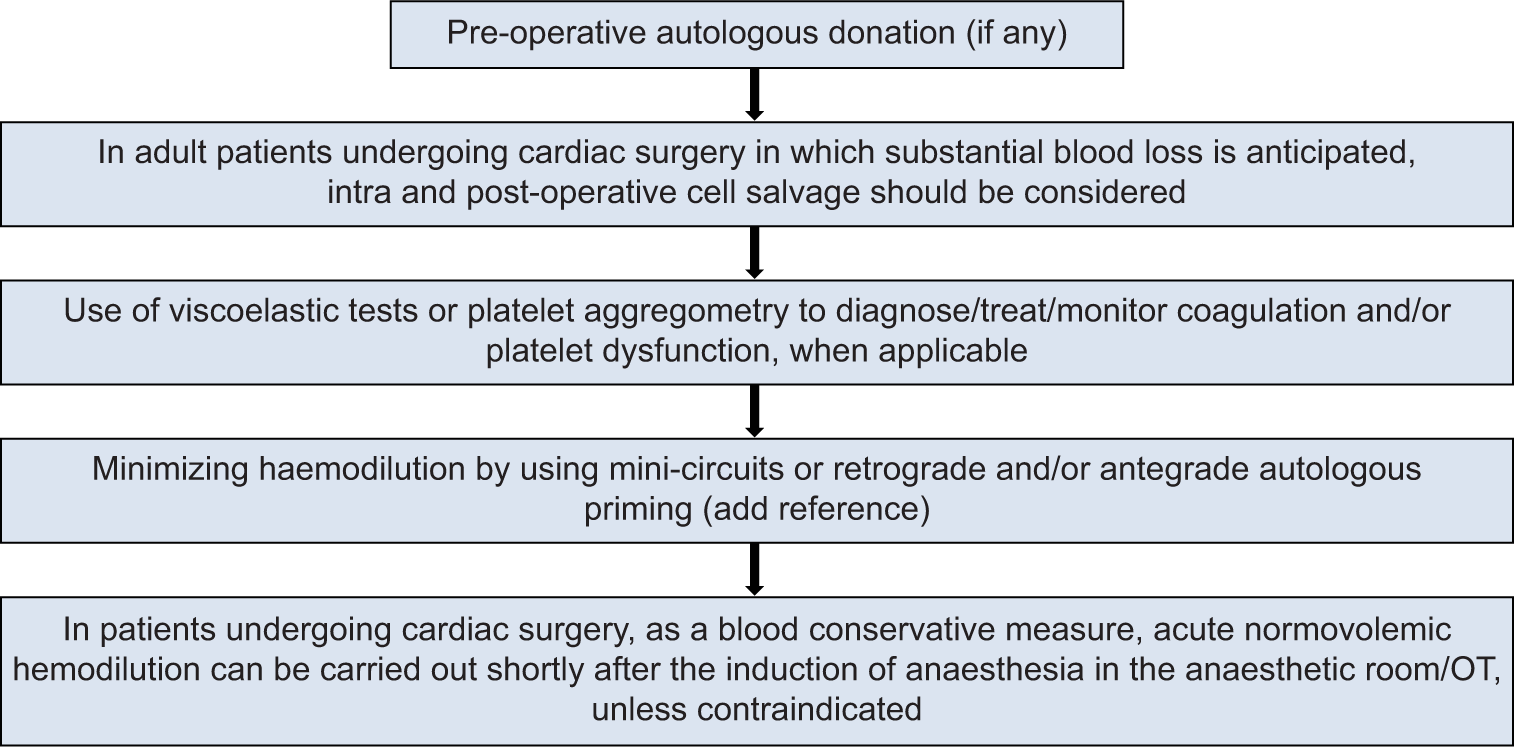
- Intraoperative pre-emptive steps to be taken by cardiac anesthesiologist on an individualized patient basis. OT: Operation Theatre.
R16. Physiological transfusion triggers: Multiple studies have proven that a restrictive blood transfusion strategy is non-inferior to liberal, thus lowering the transfusion triggers, both on-pump and off-pump. The decision to transfuse should be based on a combination of physiological transfusion triggers, reviewed with the overall clinical context (hemodynamic parameters, urine output, medical history, age, gender, etc.). It is important to avoid premature transfusion and maintain adequate tissue oxygen supply to organs at risk (85.71% Strongly agree; 14.29% Agree; SD = 0.35).
R17. In centers where viscoelastic testing is not available, the standard laboratory intraoperative blood management should be targeted toward maintaining 5 key parameters: Hemoglobin (Hb) >7.5 g/dL, platelets >50,000/microL, international normalized ratio (INR) < 1.5, fibrinogen >150 mg/dL, and ACT not more than baseline [Flowchart 2 and Box 1] (62.5% Strongly agree; 37.5% Agree; SD = 0.484).
|
| ACT: Activated clotting time, INR: International normalized ratio, CTICU: Cardiothoracic intensive care unit, CPB: Cardiopulmonary bypass, , rFVIIa: Recombinant activated factor VII |
(Hashmi NK, et al.)
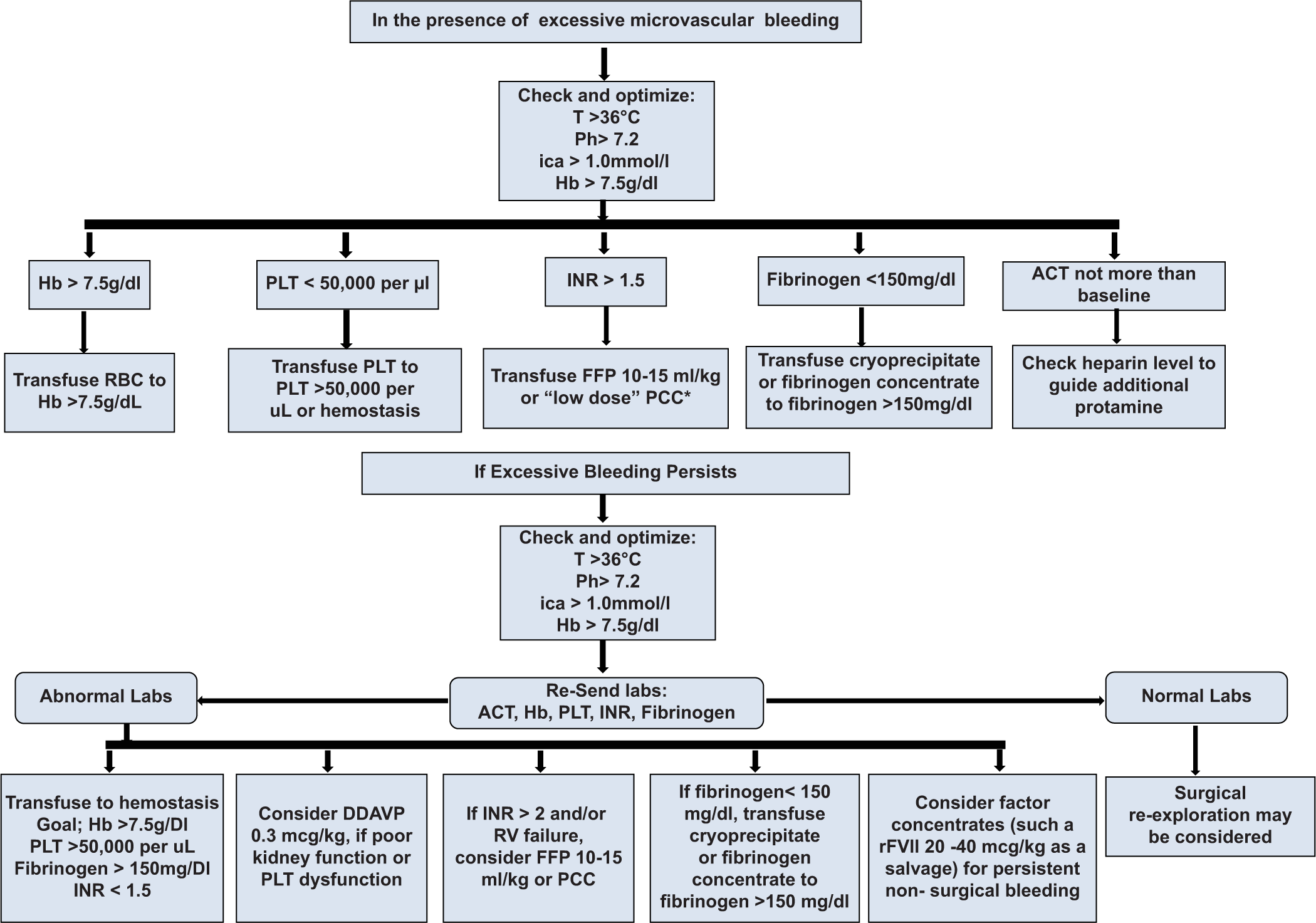
- Intraoperative algorithm for cardiac surgery targeted transfusion (Non-thromboelastography/rotational thromboelastometry directed transfusion modalities available in India). ACT: Activated clotting time, ANH: Acute normovolemic hemodilution, CPB: cardiopulmonary bypass, DDAVP: 1-deamino-8-d-arginine vasopressin, FFP: Fresh-frozen plasma, Hb: Hemoglobin, ica++: Ionized calcium, INR: International normalized ratio, PCC: Prothrombin complex concentrate, PLT: Platelets, RBC: Red blood cell, rFVIIa: Recombinant activated factor VII, RV: Right ventricle, T: Temperature.
- Source: With permission from Raphael J, et al.
To control bleeding, it is imperative to first control general physiological conditions leading to bleeding such as hypothermia, acidosis, and CPB-induced hyperfibrinolysis. The use of antifibrinolytics should be judiciously managed in the ICU as well, especially when post-protamine, excessive bleeding continues to manage microvascular bleeding, important to monitor Hb, platelets, INR, fibrinogen, and ACT as required. Most patients require a single blood component, post-investigations, but some ongoing coagulopathy patients may require more than one blood component for good hemostasis. Clinical assessment is most fundamental during ongoing component therapy.[3]
R18. When TEG ROTEM-guided transfusion protocols are followed in cardiac surgery from beginning to end, the blood transfusion quantities are diminished. The two ROTEM and TEG share a similar basic principle of viscoelastic testing with some differences in the mechanical functioning [Table 1]. The ROTEM has the cup fixed and the TEG has a cup which oscillates to a fixed pin.[15] Algorithms used for viscoelastic testing (VET) optimize need for blood transfusion even in the ICU and contribute greatly in achieving patient blood management strategies of restricted blood transfusion and avoidance of complications of blood transfusion and thus target appropriate quantity of patient care (62.5% Strongly agree; 37.5% Agree; SD = 0.484).[16]
| ROTEM | TEG | Hemostatic factors | |
|---|---|---|---|
| Clot initiation | CT (clotting time) in seconds | R (reaction time) in minutes | Enzymatic Coagulation Factors, anticoagulants, FDPs, tissue factor expression on monocytes |
| Clot kinetics | CFT in seconds; alpha angle in degrees | K (kinetic time) in min; alpha angle in degrees | Enzymatic coagulation factor, anticoagulants, fibrinogen, platelets |
| Clot strength | A5 (A10) Amplitude in min after CT in mm, MCF in mm | MA in mm | Platelets, fibrinogen, FXIII, Colloids |
| Clot stability (lysis) | LI60 (Lysis Index 60 min after CT in % of MCF | LY30 (lysis 30 min after MA) in % of MA | Fibrinolytic enzymes, fibrinolysis inhibitors, FXIII |
A5 indicates amplitude 5 min after coagulation time, A10: Amplitude 10 min after coagulation time, CFT: Clot formation time, CT: Coagulation time, MA:Maximum amplitude, MCF: Maximum clot firmness, ML: Maximum lysis during run time, ROTEM: Rotational thromboelastometry, TEG: Thrombelastography, FDPs: Fibrin degradation products
R18.1 TEG-guided bleeding management: The TEG-based values and corresponding treatment protocol are shown in Table 2 and the TEG protocol for management of bleeding during cardiac surgery is shown in Flowchart 3.[17]
| Parameter | Treatment values | Treatment protocol |
|---|---|---|
| R (TEG) | R>15 min | 2 units of FFP |
| R>10 min | FFP titrated over repeat TEG assays | |
| R<4 min | Anticoagulation | |
| R: 11–14 min | 2 units FFP | |
| R>14 min | 4 units FFP | |
| R>10 min | FFP 10 mL/kg IBW | |
| ACT (RapidTEG w/tissue factor) | Initial ACT>111–139s | Protamine 0.4 mg/kg or 2 units FFP |
| Initial ACT>140s | 2 units FFP+10-pack Cryo | |
| Subsequent ACT>110s | 2 units FFP | |
| α-angle (TEG) | α<45° | 5 units Cryo |
| α-angle (RapidTEG) | α<63° | 10-pack Cryo |
| MA (TEG) | MA<40 mm | 10 units platelets |
| MA<55 mm | 1 unit platelet apheresis | |
| MA>73 mm | Antiplatelet therapy | |
| MA: 46–54 mm | 0.3 µg/kg DDAVP | |
| MA: 41–45 mm | 1 unit platelet apheresis | |
| MA≤40 mm | 2 units of platelet apheresis | |
| MA<55 mm | 1 unit platelet apheresis | |
| MA (RapidTEG) | MA<55 mm | 1 unit platelet apheresis |
| Ly30 (RapidTEG) | Ly30≥7.5% (later reduced to≥3%) | Tranexamic acid 1 g IV |
TEG: Thrombelastography, FFP: Fresh frozen plasma, ACT: Activated clotting time, Cryo: Cryoprecipitate, MA: Maximum amplitude, DDAVP: 1-deamino-8-d-arginine vasopressin, IBW: Ideal body weight, R: Rapid
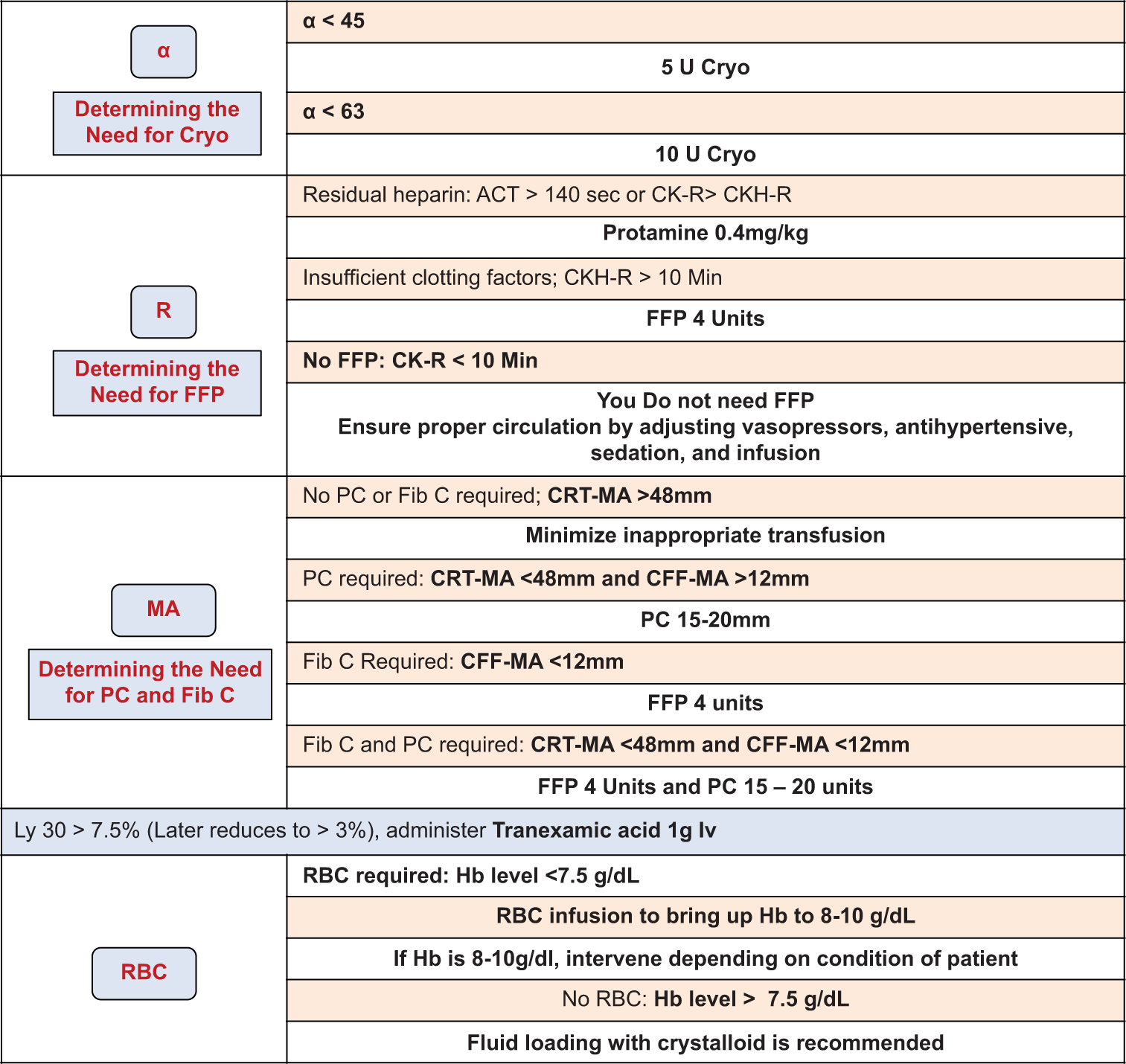
- Thrombelastography-protocol in the intensive care unit. CPB: Cardiopulmonary bypass, CBC: Complete blood count including red blood cell and platelet counts, PM: Platelet mapping by TEG6s, FibCare: A device that can rapidly measure the fibrinogen levels in the operating room, The following were the TEG6s measurement items: CK-R, CKH-R, CRT-MA, and CFF-MA. FFP: Fresh frozen plasma, MA:Maximum amplitude, RBC: Red blood cell, ACT: Activated clotting time, PC: Platelet count
R18.2 ROTEM-guided bleeding management: ROTEM results should be interpreted in a reasonable sequence (A5 FIBTEM before TEXTEM), not according to their availability (TEXTEM before A5 FIBTEM). The sequence should be guided by the clinical situation, followed by hyperfibrinolysis, followed by heparin: protamine balance, clot firmness, and then thrombin generation. This avoids potential misinterpretation of ROTEM results. The TEG/ROTEM-based intraoperative blood management is shown in Flowchart 4 for adult cardiac surgery and Flowchart 5 for congenital cardiac conditions, supported by considerations in Box 2. These flowcharts should be implemented in coordination with the entire multidisciplinary team at the ground level.[18]
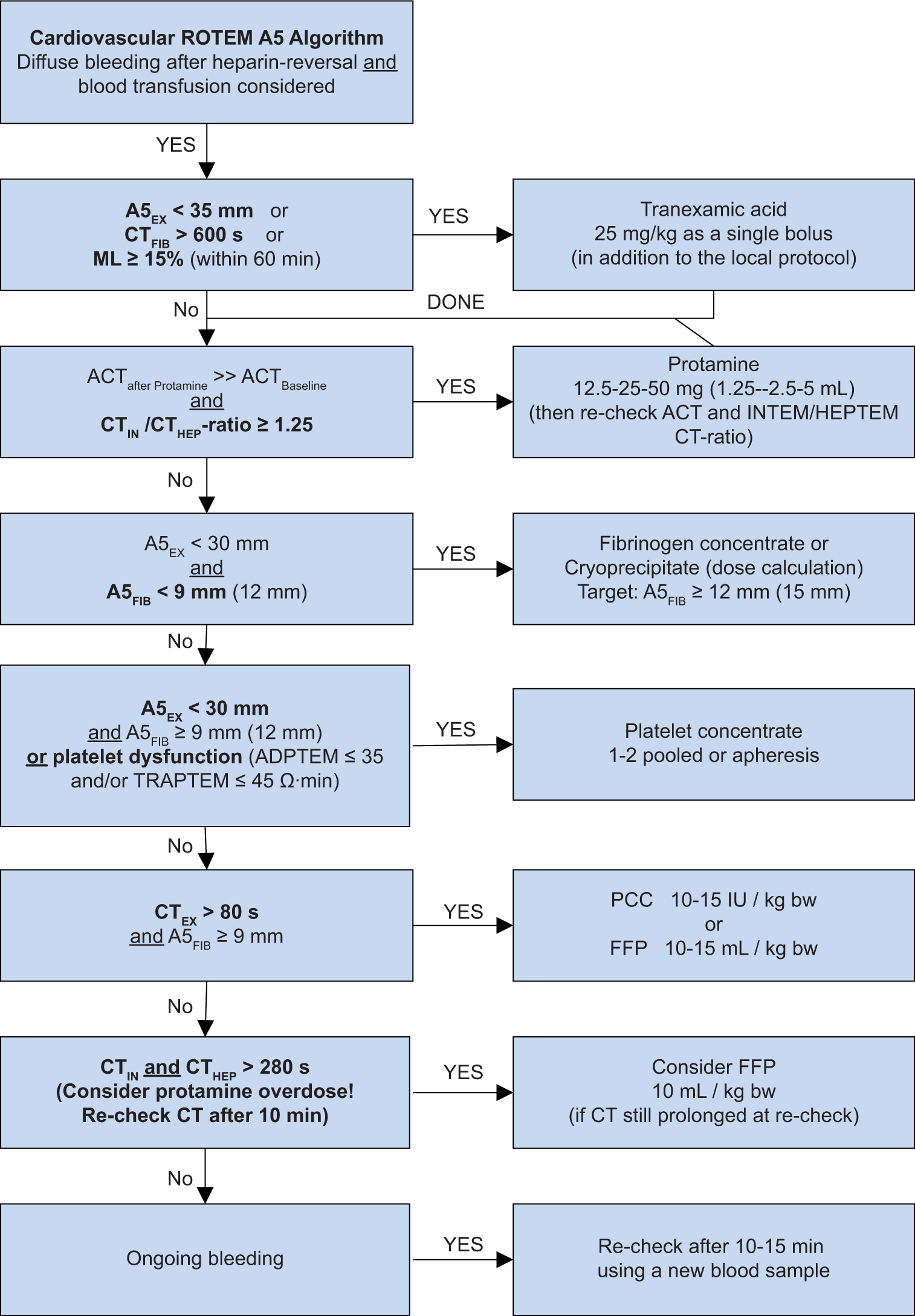
- Transfusion algorithm during cardiac surgery (Point-of-care testing based). FFP: Fresh frozen plasma, CT: Clotting time, PCC: Prothrombin complex concentrate, TRAPTEM: Thrombin receptor activating peptide-6 assay of ROTEM platelet, ADPTEM:Adenosine diphosphate based thromboelastometry test, INTEM: Intrinsically activated thromboelastometric test, HEPTEM: Heparinase assay based thromboelastometry, ACT: Activated clotting time, ML: Maximum lysis
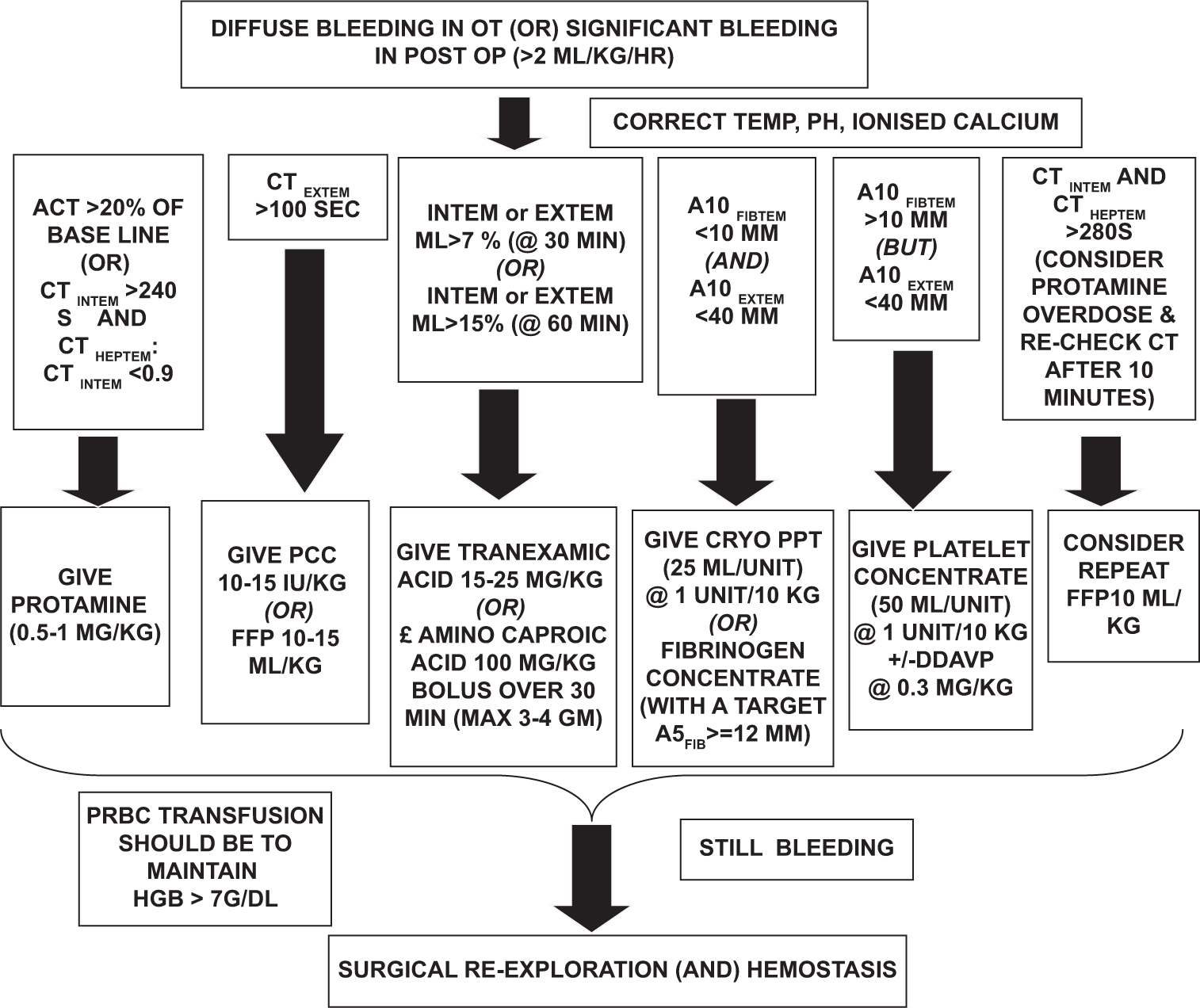
- Rotational thromboelastometry protocol as followed in All India Institute of Medical Sciences, for bleeding management in cyanotic congenital heart disease surgical patients. OT: Operation theatre, OP: Operative HGB: Haemoglobin, FFP: Fresh Frozen Plasma, CT: Clotting Time, PCC: Prothrombin Complex Concentrate, INTEM: Intrinsically activated thromboelastometric test, HEPTEM: Heparinase assay based thromboelastometry, ACT: Activated Clotting Time, EXTEM:Extrinsic thromboelastometry
|
| ROTEM: Rotational thromboelastometry, TEG: Thrombelastography, ANH: Acute normovolemic hemodilution |
(Hashmi NK, et al.)
Indian pediatric cardiac surgical studies[19] have shown that algorithm-based blood component therapy led to significantly lower incidence of PRBCs, FFP, platelets, and cryoprecipitate administered and significantly shorter duration of mechanical ventilation, duration of ICU stay, and hospital stay. The Flowchart 5 has been adapted from same study.
R18.3 Targeting platelet aggregation is essential for patients on antiplatelet therapies, including acetylsalicylic acid, P2Y12 adenosine diphosphate receptor blockers, and glycoprotein IIb/IIIa inhibitors. Platelet aggregometry tests are effective for detecting and managing platelet-related bleeding in cardiac surgery.[15,20-32]
R19. All cardiac anesthetists who manage cardiac surgical patients should not only have knowledge of but also be experienced and well-versed with both approaches as mentioned in R17 and R18, that is, both with and without viscoelastic testing facilities available. More so, viscoelastic tests are performed at the bedside, have a short turnaround time, and guide clinicians toward a more goal-directed transfusion management, and hence this working group recommends that more Indian institutes invest in its infrastructure and technical training for viscoelastic monitoring[33,34] (85.71% Strongly agree; 14.29% Agree; SD = 0.433).
Post-operative recommendations
R20. The etiology of excessive blazing is generally due to technical regions, 74% cases ongoing in coagulopathy, 30% combination of both, and 10% cases and miscellaneous others in 3% patients.[12] The need for blood transfusion is felt maximum when the patient has one of the following parameters: Assessment of fluid balance, fluid resuscitation, and bleeding, weaning failure from mechanical ventilation, high doses of vasopressor, and signs of severe ventricular dysfunction[3,35] (75% Strongly agree; 25% Agree; SD = 0.433).
R21. Post-CPB bleeding management is best followed by the Kirklin and Barratt-Boyes criteria,[36] which circulates: the following – (1) when there is drainage as shown in Table 3 and Box 3 (2) when excessive bleeding suddenly restarts indicating a surgical cause or (3) presence of sudden massive bleeding (85.71% Strongly agree; 14.29% Agree; SD = 0.35).
| 1 | Haemoglobin (Hb) | → | Restrictive RBC transfusion threshold of Hb 7.5 g/dL is recommended in stable patients,(TRICS trial) whereas patients undergoing high-risk cardiac surgery (ongoing ischaemia, CVA, history of CHF, microvascular bleeding), should be transfused to an Hb of 8-10 g/dL. RBCs should not be transfused at an Hb ≥10 g/dL, with the potential exception of univentricular paediatric cardiac surgery patients. Hb level alone should not dictate transfusion but it should also be based on multiplicity of clinical factors such as rate of Hb decline, cardiopulmonary reserve, amount of acute blood loss being ≥15% of total blood volume, etc. Recently collected RBCs (≤5 days old) should be used in children and neonates and also in adults with end-stage renal disease. |
| 2 | Haematocrit | → | A haematocrit of 21–24% may be considered during CPB when an adequate DO2(>273 ml O2/min/m2) level is maintained |
| 3 | Tranexamic acid or EACA infusion | → | Tranexamic acid or EACA infusion significantly reduces the proportion of patients who receive allogeneic red blood cell transfusion |
RBC: Red blood cells, Hb: Haemoglobin, EACA: Epsilon aminocaproic acid, CPB: Cardiopulmonary bypass, CVA: Cerebrovascular accident, CHF: Congestive heart failure, DO2: Oxygen delivery.
| Volume of blood drainage | Time from surgery |
|---|---|
| >500 mL | During the 1sth |
| >400 mL | During the next 2 h |
| >300 mL | During each of the first 3 h |
| >1000 mL | In total 4 h |
| >1200 mL | In total 5 h |
R22. Implement restrictive transfusion trigger just like pre- and intra-operative setting and optimize with correction/optimization of hypothermia, normalize acid-base status and electrolytes, ionized calcium, Mean arterial pressure of 60–75 mm Hg, Positive End-Expiratory Pressure 8–10 cm, checking for chest tube patency, heparin rebound (ACT of 120–140s), Activated Partial Thromboplastin Time, prothrombin time, fibrinogen levels, platelet count, factors, and hyperfibrinolysis. In addition, one must employ anemia tolerance, reduce oxygen consumption, optimize pain control, avoid tachycardia and hypertension, and avoid unnecessary transfusion (top-up transfusion)[37] (100% Strongly agree; SD = 0).
R23. The treatment options used to manage post-operative bleeding are protamine to treat heparin rebound, PRBC, cryoprecipitate, platelet concentrate (random donor/single donor), FFP, fibrinogen concentrate, PCC, tranexamic acid, EACA, desmopressin, and recombinant factor VIIa[38] (77.78% Strongly agree; 22.22% Agree; SD = 0.416).
R24. The transfusion thresholds include PRBC (Hb < 7.5 g/L), platelets (<100 × 103), PCC (INR > 1.5), FFP (INR > 1.5), and fibrinogen (<1.5 g/L)[39] (75% Strongly agree; 25% Agree; SD = 0.433).
R25. Recombinant activated factor VII (rFVIIa) is used as a last resort to treat coagulopathy. rFVIIa should not be used to buy time before re-exploration[40] (57.14% Strongly agree; 42.86% Agree; SD = 0.495).
R26. Topical application of antifibrinolytic agents or fibrin sealant to the surgical site intra operatively is reasonable to reduce blood loss and transfusion requirements[41] (71.43% Strongly agree; 28.57% Agree; SD = 0.452).
DISCUSSION
The World Health Organization has emphasized on the urgency of implementing patient blood management across all surgical procedures.[42] Our recommendations are in line with those and other international recommendations for bleeding management[43-47] and have several strengths as well as limitations as mentioned in Table 4.
| Strengths | Limitations |
|---|---|
|
|
The working group emphasizes that hospital protocols be tailored to available therapeutic options and evidence-based patient blood management lead to better outcomes in cardiac surgeries. As new priorities, infrastructure, treatments, and evidence emerge, revising this consensus will be necessary. Indian hospital protocols should be reviewed annually, considering recent evidence and available blood products, including new factor concentrates. Establishing centers of excellence at key hospitals can help standardize coagulation management and train peripheral centers using a “hub-and-spoke” model. Enhancing the visibility of hospital-based algorithms through mobile apps, websites, or printed stickers can support decision-makers in managing bleeding during cardiac surgery. In addition, efforts must be made to educate anesthesiologists and cardiac surgeons about new factor concentrates, addressing knowledge gaps from medical training.[48-51]
CONCLUSION
Bleeding during cardiac surgery requires a comprehensive, multidisciplinary approach. Limited Indian guidelines, along with constraints in evidence, resources, time, and reimbursement, impede standardized management. This E-Delphi method has, for the first time, produced Indian expert-derived consensus recommendations for managing bleeding in cardiac surgeries. These recommendation statements aim to optimize management approaches, potentially setting a new standard of care in India and beyond.
Acknowledgment
The authors acknowledge the efforts of Dr. Pratishtha Chaudhari for her invaluable support in medical writing, adhering to Good Publication Practice (GPP) guidelines, collating E-Delphi results, and providing essential liaison and coordination assistance for the consensus development. In addition, Dr. Saurabh Suri, a consultant anesthesiologist, shared his expert insights that greatly informed the medical writing process.
Ethical approval:
The Institutional Review Board approval is not required.
Declaration of patient consent:
Patient’s consent not required as there are no patients in this study.
Conflicts of interest:
This is to declare that Dr. Poonam Malhotra Kapoor, Dr. Naman Shastri, Dr. Muralidhar Kanchi, and Dr. Yatin Mehta are on the editorial board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship:
The E-Delphi subscription (3 months) and the medical writing support was funded by CSL Behring Pte. Ltd. However, the sponsor had no role in the Delphi process and influence its decision, which involved concept/design, participation in steering committee, execution of the Delphi process, analysis of study findings, development, and submission of the paper for publication. The sponsor did support in organizing the three meetings (one in-person meeting and two virtual meetings).
References
- Equal to the Challenge Cardiac Surgery in India-As Many Opportunities as there are Challenges. Indian J Thorac Cardiovasc Surg. 2023;39:222-30.
- [CrossRef] [PubMed] [Google Scholar]
- Costs of Excessive Postoperative Hemorrhage in Cardiac Surgery. J Thorac Cardiovasc Surg. 2009;138:687-93.
- [CrossRef] [PubMed] [Google Scholar]
- Active Bleeding after Cardiac Surgery: A Prospective Observational Multicenter Study. PLoS One. 2016;11:e0162396.
- [CrossRef] [PubMed] [Google Scholar]
- Reexploration for Hemorrhage Following Coronary Artery Bypass Grafting: Incidence and Risk Factors. Northern New England Cardiovascular Disease Study Group. Arch Surg. 1998;133:442-7.
- [CrossRef] [PubMed] [Google Scholar]
- Reoperation for Bleeding in Cardiac Surgery. Interact Cardiovasc Thorac Surg. 2012;14:709-13.
- [CrossRef] [PubMed] [Google Scholar]
- Hypothermia-Induced Coagulopathy During Hemorrhagic Shock. Am Surg. 2000;66:348-54.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Hypothermia on the Coagulation Cascade. Crit Care Med. 1992;20:1402-5.
- [CrossRef] [PubMed] [Google Scholar]
- Hypothermia and Acidosis Worsen Coagulopathy in the Patient Requiring Massive Transfusion. Am J Surg. 1990;160:515-8.
- [CrossRef] [PubMed] [Google Scholar]
- Reexploration for Bleeding After Coronary Artery Bypass Surgery: Risk Factors, Outcomes, and the Effect of Time Delay. Ann Thorac Surg. 2004;78:527-34.
- [CrossRef] [PubMed] [Google Scholar]
- Resternotomy for Bleeding after Cardiac Operation: A Marker for Increased Morbidity and Mortality. Ann Thorac Surg. 1995;59:664-7.
- [CrossRef] [PubMed] [Google Scholar]
- Universal Definition of Perioperative Bleeding in Adult Cardiac Surgery. J Thorac Cardiovasc Surg. 2014;147:1458-63.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Current Guidelines for Blood Conservation in Cardiac Surgery. Indian J Anaesth. 2009;53:265-7.
- [Google Scholar]
- Patient Blood Management in Cardiac Surgery and ECMO: The Indian Scenario in 2021. J Cardiac Crit Care TSS. 2021;5:181-3.
- [CrossRef] [Google Scholar]
- ACCORD (ACcurate COnsensus Reporting Document): A Reporting Guideline for Consensus Methods in Biomedicine Developed Via a Modified Delphi. PLoS Med. 2024;21:e1004326.
- [CrossRef] [PubMed] [Google Scholar]
- Role of Platelet Function Test in Predicting Postoperative Bleeding Risk after Coronary Artery Bypass Grafting: A Prospective Observational Study. J Cardiac Crit Care TSS. 2021;5:88-96.
- [CrossRef] [Google Scholar]
- Targeted Coagulation Management in Severe Trauma: The Controversies and the Evidence. Anesth Analg. 2016;123:910-24.
- [CrossRef] [PubMed] [Google Scholar]
- Review of Thromboelastography (TEG): Medical and Surgical Applications. Ther Adv Pulm Crit Care Med. 2023;18:29768675231208426.
- [CrossRef] [PubMed] [Google Scholar]
- The Role of Evidence-based Algorithms for Rotational Thromboelastometry-guided Bleeding Management. Korean J Anesthesiol. 2019;72:297-322.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital Cyanotic Cardiac Surgery in Children: Is Algorithm-Based Point-of-Care Testing Essential to Prevent Bleeding? J Cardiac Crit Care TSS. 2019;2:84-90.
- [Google Scholar]
- Utility of Platelet Function Tests: A Recent Review Round Up. J Card Crit Care TSS. 2020;3:24-7.
- [CrossRef] [Google Scholar]
- Platelet Function Test in Coronary Artery Bypass Grafting: Does It Predict Postoperative Bleeding? J Cardiac Crit Care TSS. 2021;5:186-95.
- [CrossRef] [Google Scholar]
- Massive Transfusion/Hemorrhage Protocols Versus Goal-Directed Bleeding Management: Science Gone Eerie? J Cardiac Crit Care TSS. 2024;8:16-27.
- [CrossRef] [Google Scholar]
- Patient Blood Management: Moving Above and Beyond the Optimal Use of Blood! J Card Crit Care TSS. 2024;8:28-32.
- [CrossRef] [Google Scholar]
- Thromboelastography in Venovenous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. J Cardiac Crit Care TSS. 2017;1:101-4.
- [CrossRef] [Google Scholar]
- Is Rotational Thromboelastometry the Answer for Rapid Prediction of Coagulopathy on Extracorporeal Membrane Oxygenation? J Cardiac Crit Care TSS. 2017;1:108-10.
- [CrossRef] [Google Scholar]
- Anticoagulation During ECMO: Will the Tight Rope be Tighter in 2018? J Cardiac Crit Care TSS. 2017;1:55-6.
- [CrossRef] [Google Scholar]
- Prospective Interventional Cohort Study Using AIIMS Simplified POC Algorithm for Restricted Blood Transfusion in Cyanotic Children. J Card Crit Care TSS. 2024;8:195-204.
- [CrossRef] [Google Scholar]
- Value of Point-of-Care Algorithms in Pediatric Cardiac Surgery. J Card Crit Care TSS. 2024;8:181-4.
- [CrossRef] [Google Scholar]
- Viscoelastic Testing on Venoarterial Extracorporeal Membrane Oxygenation: Need or Greed? J Card Crit Care TSS. 2023;7:118-28.
- [CrossRef] [Google Scholar]
- Platelet Reactivity on ECMO: Role of Verify Now. J Card Crit Care TSS. 2023;7:129-32.
- [CrossRef] [Google Scholar]
- A Prospective Randomized Clinical Trial of Efficacy of Algorithm-Based Point of Care Guided Hemostatic Therapy in Cyanotic Congenital Heart Disease Surgical Patients. J Card Crit Care TSS. 2019;3:8-16.
- [CrossRef] [Google Scholar]
- Reexploration Can Be Deterred by Point-of-Care Testing in Cardiac Surgery Patient. J Card Crit Care TSS. 2017;1:48-50.
- [CrossRef] [Google Scholar]
- Are Viscoelastic Tests Clinically Useful to Identify Platelet-Dependent Bleeding in High-Risk Cardiac Surgery Patients? Anesth Analg. 2022;135:1198-206.
- [CrossRef] [PubMed] [Google Scholar]
- The Effectiveness of 10 Years of Interventions to Control Postoperative Bleeding in Adult Cardiac Surgery. Interact Cardiovasc Thorac Surg. 2017;24:196-202.
- [CrossRef] [PubMed] [Google Scholar]
- Causes of Excessive Bleeding in Patients Who Underwent Open-Heart Surgery During the Early Postoperative Period. GKDA Derg. 2024;30:1-8.
- [CrossRef] [Google Scholar]
- Kirklin/Barratt-Boyes: Cardiac Surgery (4th ed). Netherlands: Elsevier; 2013. p. :2256.
- [Google Scholar]
- Effect of a Restrictive vs Liberal Blood Transfusion Strategy on Major Cardiovascular Events Among Patients With Acute Myocardial Infarction and Anemia: The REALITY Randomized Clinical Trial. JAMA. 2021;325:552-60.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac Surgery and Blood-Saving Techniques: An Update. Cureus. 2022;14:e21222.
- [CrossRef] [Google Scholar]
- Transfusion Thresholds in Cardiac Surgery: Commentary on Bracey et al., 1999. Transfusion. 2022;62:2438-48.
- [CrossRef] [PubMed] [Google Scholar]
- Off-label Use of Recombinant Activated Factor VII for Cardiac Surgical Bleeding. Anesthesiology. 2023;139:197-210.
- [CrossRef] [PubMed] [Google Scholar]
- Topical Application of Antifibrinolytic Drugs for On-pump Cardiac Surgery: A Systematic Review and Meta-analysis. Can J Anaesth. 2009;56:202-12.
- [CrossRef] [PubMed] [Google Scholar]
- The Urgent Need to Implement Patient Blood Management: Policy Brief. 2021. Geneva: WHO; https://www.who.int/publications/i/item/9789240035744 [Last accessed on 2024 Jun 28]
- [Google Scholar]
- 2021 Clinical Practice Guidelines for Anesthesiologists on Patient Blood Management in Cardiac Surgery. J Cardiothorac Vasc Anesth. 2021;35:3493-5.
- [CrossRef] [PubMed] [Google Scholar]
- STS/SCA/AmSECT/SABM Update to the Clinical Practice Guidelines on Patient Blood Management. J Extra Corpor Technol. 2021;53:97-124.
- [CrossRef] [PubMed] [Google Scholar]
- ERAS Cardiac Society Turnkey Order Set for Patient Blood Management: Proceedings from the AATS ERAS Conclave 2023. J Thorac Cardiovasc Surg. 2024;168:890-7.e4.
- [CrossRef] [PubMed] [Google Scholar]
- Management of Severe Peri-operative Bleeding: Guidelines from the European Society of Anaesthesiology and Intensive Care: Second Update 2022. Eur J Anaesthesiol. 2023;40:226-304.
- [CrossRef] [PubMed] [Google Scholar]
- Data and Metrics for Patient Blood Management: A Narrative Review and Practical Guide. Anesth Analg 2023
- [CrossRef] [PubMed] [Google Scholar]
- Implementation of Patient Blood Management-A Long and Winding Road but Worth Doing! J Card Crit Care TSS. 2024;8:1-4.
- [CrossRef] [Google Scholar]
- New Horizons for Reduction of Blood Use: Patient Blood Management. Asian J Transfus Sci. 2023;17:108-16.
- [CrossRef] [PubMed] [Google Scholar]
- Three-factor prothrombin complex concentrates for refractory bleeding after cardiovascular surgery within an algorithmic approach to haemostasis. Vox Sang. 2019;114:374-385.
- [CrossRef] [PubMed] [Google Scholar]
- Society of Cardiovascular Anesthesiologists Clinical Practice Improvement Advisory for Management of Perioperative Bleeding and Hemostasis in Cardiac Surgery Patients. J Cardiothorac Vasc Anesth. 2019;33:2887-99.
- [CrossRef] [PubMed] [Google Scholar]







