Translate this page into:
A Retrospective Observational Study in Neonates with Transposition of Great Arteries Undergoing Balloon Atrial Septostomy in a Tertiary Care Hospital
*Corresponding author: Mohanish Badge, Department of Cardiac Anaesthesia and Critical Care, All India Institute of Medical Sciences, New Delhi, India. mohanish.badge11@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Badge M, Choudhury M, Pallavi B. A Retrospective Observational Study in Neonates with Transposition of Great Arteries Undergoing Balloon Atrial Septostomy in a Tertiary Care Hospital. J Card Crit Care TSS. 2024;8:39-44. doi: 10.25259/JCCC_24S1_MB
Abstract
Objectives:
Transposition of great arteries (TGA) is a common cause of cyanotic newborns. There is an atrioventricular concordance with ventriculoarterial discordance. This parallel circulation is incompatible with life unless communication exists for the inter-circulatory mixing of blood. Balloon atrial septostomy (BAS) is a percutaneously performed interventional procedure in catheterization laboratory, usually in patients with TGA-intact ventricular septum (IVS) to ensure admixture of oxygenated and deoxygenated blood thus improving systemic oxygen delivery, to achieve hemodynamic stability before the definitive procedure (Arterial switch operation), and to determine the risk of intraprocedural complications in neonates undergoing balloon atrial septostomy.
Material and Methods:
This is a retrospective observational study, which included neonatal patients during a single year. All the data were collected from the medical record section of the hospital.
Results:
We included 17 neonates with TGA transferred to our center for definite treatment. Six cases were done under sedation and 11 under general anesthesia. The mean age at the time of BAS was 4.8 days. Procedure-related complications occurred in 41% of patients. In one of the cases, difficult airway management made the periprocedural course daunting. Complications included intraprocedural balloon rupture (1 case), transient atrial arrhythmia (4 cases), hypotension (1 case), and pericardial tamponade due to left atrial wall puncture (1 case).
Conclusion:
BAS is a safe and effective palliative procedure for TGA-IVS, with good immediate results in our institution. Maintaining cardiorespiratory stability, prevention of respiratory depression in a spontaneously breathing neonate, and maintenance of normothermia in the cold temperature of the catheterization laboratory, with eternal vigilance, forms the cornerstone of a successful neonatal outcome.
Keywords
Neonates
Transposition of great arteries
Undergoing balloon atrial septostomy
Congenital heart defects
Transposition of great arteries
INTRODUCTION
Transposition of great arteries (TGA) is the most common cause of a cyanotic newborn, accounting for 5–7% of all congenital heart defects. There is an atrioventricular concordance with ventriculoarterial discordance. That is, the aorta arises from the right ventricle, supplying deoxygenated blood to the systemic circulation, and the pulmonary artery from the left ventricle supplies oxygenated blood to pulmonary circulation. This anatomical configuration results in a parallel circulation, with resulting hypoxemia, lactic acidosis, and death, unless a communication exists for inter-circulatory mixing of blood at two of the three sites, namely, atrial septal defect (ASD)/patent foramen ovale (PFO), ventricular septal defect (VSD), or patent ductus arteriosus. Without intervention, this disease has a high mortality rate with 45% deaths in the first month of life. Balloon atrial septostomy (BAS), originally described by Rashkind and Miller in 1966, is a percutaneously performed interventional procedure in a cardiac catheterization laboratory in patients with TGA-intact ventricular septum (TGA-IVS) with small PFO/ restrictive ASD to ensure admixture of oxygenated and deoxygenated blood and to achieve hemodynamic stability before the definitive surgical procedure (Arterial Switch Operation).[1] BAS requires cannulation of the usually femoral vein (Umbilical vein can be used) with a 5-Fr pediatric sheath, through which a balloon is passed to cross the interatrial septum; the balloon is inflated in the left atrium with dilute contrast and sharply pulled back to produce a tear in the interatrial septum (IAS), increasing the interatrial communication size. It can be repeated until the desired effect is obtained. It is done under cinefluroscopic and transthoracic echocardiographic guidance. Immediate improvement in saturation with echocardiographic flow across the IAS confirms the successful completion of the procedure. The procedure itself has its complications, along with the challenging anesthesia requirement of a non-operating room setting and a sick neonate.
Aim of study
This study aimed to determine the risk of intraprocedural complications in neonates undergoing BAS in a tertiary care hospital.
Study design
This was a retrospective observational study.
MATERIAL AND METHODS
We conducted a single-center, observational, and retrospective study between January 2022 and December 2022.
Inclusion criteria
The following criteria were included in the study:
BAS performed for TGA-IVS with small PFO/restrictive ASD
Age <28 days
Exclusion criteria
The following criteria were excluded from the study:
Patients older than one month of age
BAS performed for other diagnoses, for example, hypoplastic left heart syndrome and total anomalous pulmonary venous connection
TGA with VSD
Data collection
The ethical clearance was obtained from the Institute Ethical Committee. All the data were collected from the patient’s record. A retrospective review of medical records of patients with TGA-IVS was done. The record of patients undergoing BAS for one year between (January 2022 and December 2022) was checked, and all neonatal patients satisfying inclusion criteria were inducted into the study. All the data were collected from the indoor files, stored in the central record room of the hospital. Data collection included age, weight at the time of presentation, gender, prenatal diagnosis of TGA, prostaglandin E1 (PGE1) infusion, place of performance, planned or emergency procedure, mode of anesthesia, difficult airway, and procedural complication.
Statistical analysis
Statistical analysis of data was done using the Statistical Package for the Social Sciences (SPSS software version 27 for Windows, Chicago, IL). The descriptive statistical analysis of the quantitative variables was carried out by calculating the mean and standard deviations. The qualitative data obtained were expressed in percentages.
RESULTS
There were 17 patients satisfying the inclusion criteria of the study, all were transferred to our center for definitive treatment. All cases (n = 17) were done as emergency procedures, and the interventions were performed in the cardiac catheterization laboratory. None of the patients was diagnosed with TGA prenatally. The mean (± standard deviation [SD]) age of the patients was 4.8 (± 2.309 days), with the youngest being two days and the oldest 14 days neonate. Thus, all patients were <15 days of age. The mean (± SD) weight of the patient was 2.82 (± 0.29) kilograms (kg), the lowest being 2.8 kg, and the highest was 3.9 kg. The number of male (n = 11, 65%) neonates was more than females (n = 6, 35%). The patient demographic data are summarized in Table 1.
| Age (days) | 4.8±2.309 (Mean±SD) |
|---|---|
| Weight (kg) | 2.82±0.29 (Mean±SD) |
| Gender (number) | Male (11) Female (6) |
| Gender (percentage) | Male (65) Female (35) |
SD: Standard deviation
Twelve (70%) of the 17 patients were on PGE1 infusion at the time of presentation to the catheterization laboratory [Figure 1]. The anesthetic technique used in 6 (35%) patients was intravenous sedation, whereas 11 (65%) patients received general anesthesia with endotracheal intubation [Figure 2]. Difficult airway was encountered in 1 (9%) out of the 11 intubated patients.
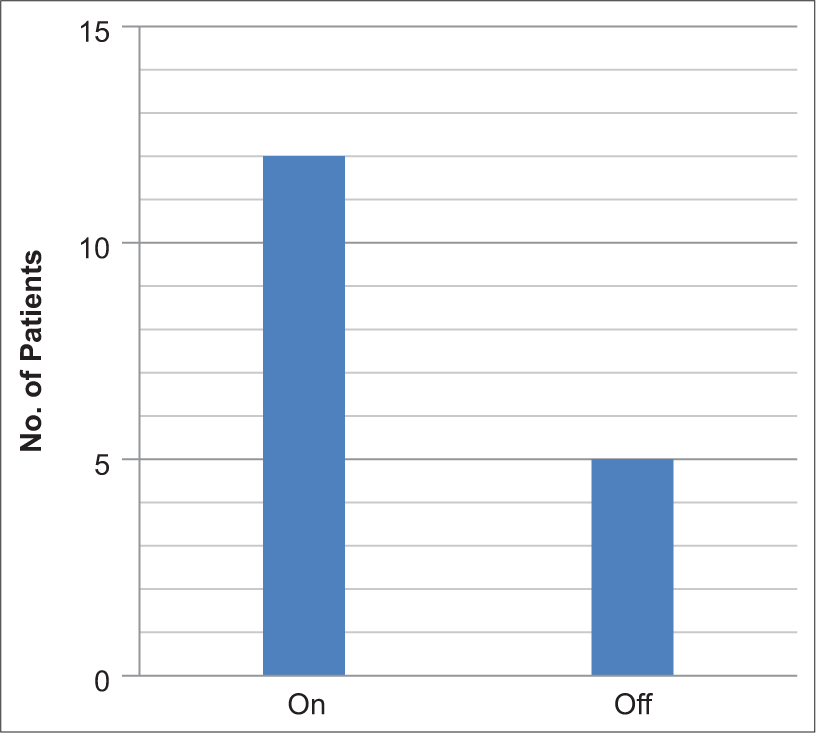
- Prostaglandin E1 infusion incidence.
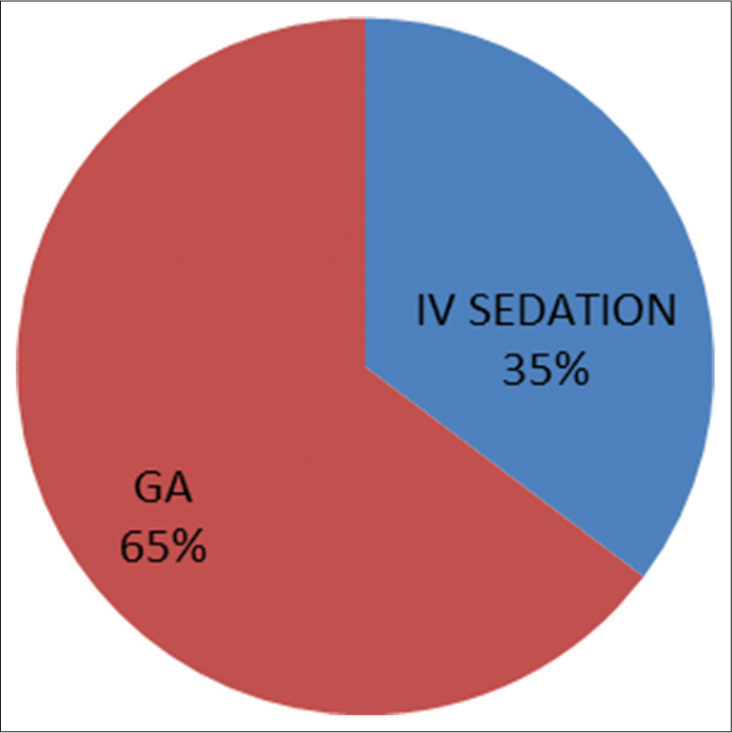
- Mode of anesthesia (IV: intravenous, GA: general anesthesia).
Procedure-related complications occurred in 7 (41%) patients [Figure 3], with transient atrial arrhythmias being the commonest in 4 (23%), followed by an equal incidence of 5% balloon rupture, hypotension, and pericardial tamponade due to left atrial wall puncture in one case requiring emergent surgical intervention. There was no case of stroke, femoral vascular thrombosis, balloon fragment embolization, or mitral valve damage [Table 2 and Figure 4]. There was no intraprocedural mortality. The procedure was uneventful in 10 (59%) patients.
| Complication | Incidence |
|---|---|
| Balloon rupture | 1 |
| Hypotension | 1 |
| Embolization of balloon fragments | 0 |
| Atrial arrhythmias | 4 |
| Pericardial effusion/cardiac puncture | 1 |
| Mitral valve damage | 0 |
| Femoral vein thrombosis | 0 |
| Femoral access site bleeding | 0 |
| Mortality | 0 |
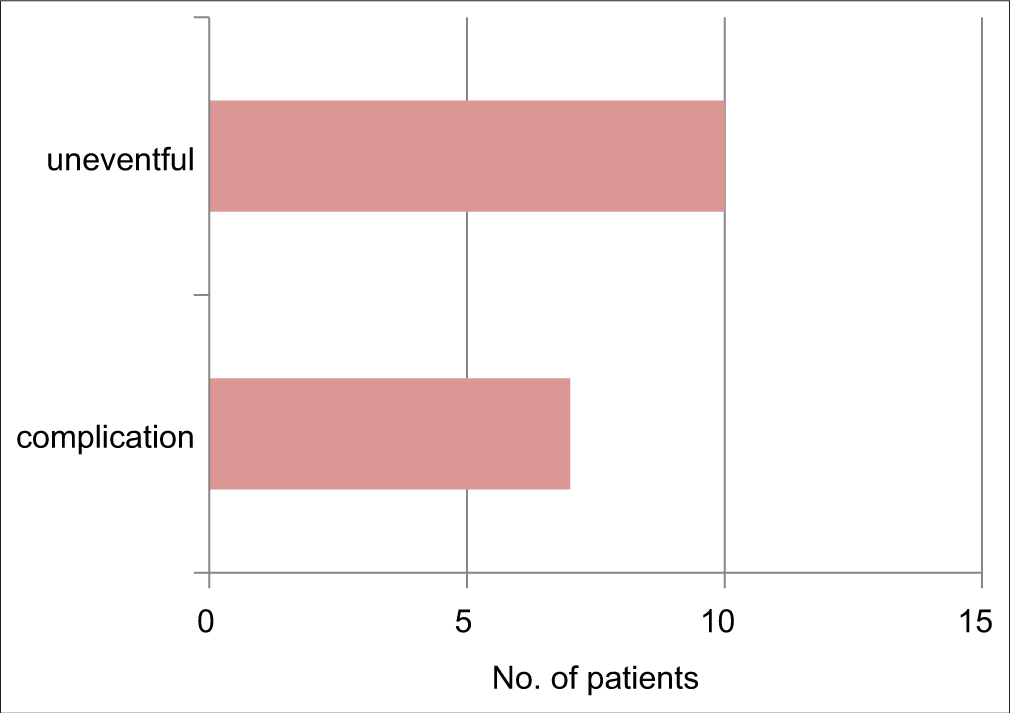
- Overall complication of the procedure.
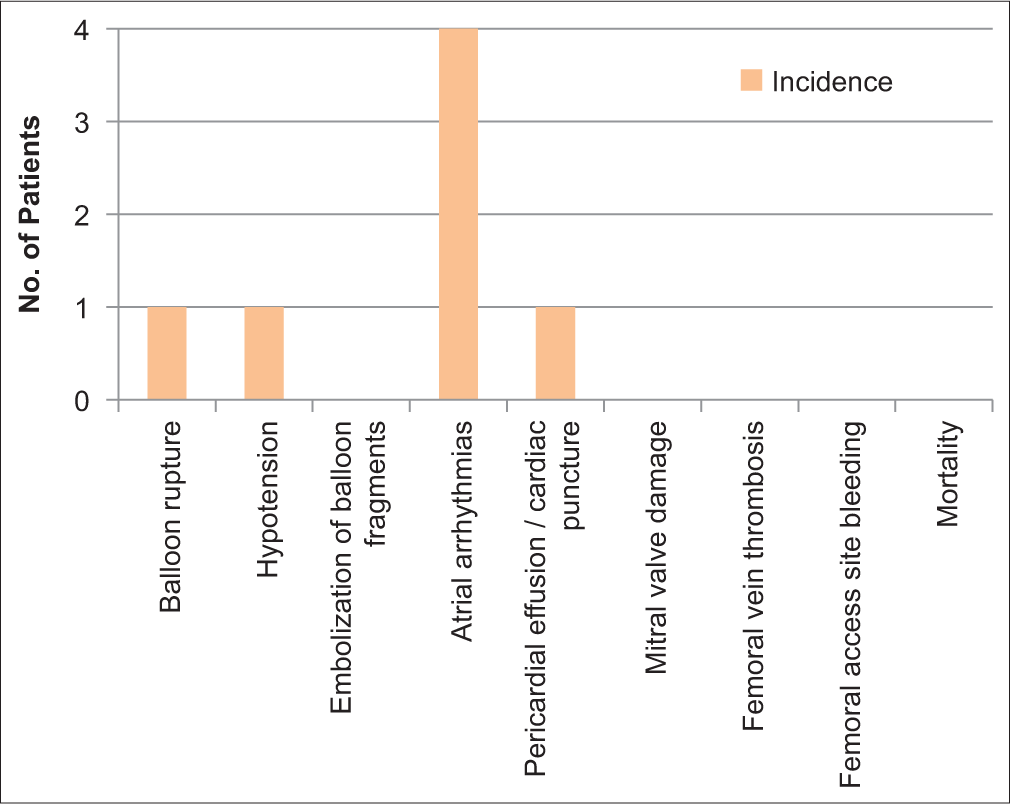
- Distribution of complications.
DISCUSSION
BAS is a life-saving procedure for patients born with TGA. Creating a non-restrictive atrial communication optimizes mixing at the atrial level, increases systemic arterial oxygenation cardiac output, as well as decreases the left atrial pressures. These beneficial effects often lead to the stabilization of these neonates and improve their condition before they undergo an operation.[2]
Our study revealed that the mean age of the patients on the day of the procedure was 4.8 ± 2.309 days, with an age range from 2 to 14 days. Joutey et al., found an age range from 5 to 75 days, and the mean age was 25 days. It included patients beyond the neonatal group.[3] Various published studies show a wide range of ages of patients undergoing BAS. The reason for this wide age range can be related to the late presentation and diagnosis of patients, along with our study population being limited to neonates.
The mean weight in our patients was 2.82 ± 0.29 kg. The average weight of patients in a study by Al-Kassmy et al., in a Canadian population was 2.40 ± 0.71 kg, which was nearer to our patient population.[4] In an Iraqi population retrospective study of 56 patients, the mean weight at the time of the procedure was 3.04 kg.[5]
There was male predominance in our study, with male newborns accounting for 65%, compared to 35% of female patients. The ratio of male to female was approximately 2:1. This has been found in many population studies. Cirstovenu et al.[6], in a comparison of neonates with TGA between BAS and non-BAS groups, found male patients exceeded the number of females in both groups. The BAS group (n = 25) had 10 (40%) females compared to 15 (60%) male neonates. A similar finding was noted by Soongswang et al.[7]
Paucity of mass programs for fetal screening in a large population with varied socioeconomic status like us; majority of TGA’s are usually diagnosed postnatally. Our study had no prenatally diagnosed TGA, echoing the condition in the majority of other countries.[7] Prenatal diagnosis of TGA was shown to be helpful in reducing early mortality by minimizing the time required for BAS.[8]
Prostaglandin therapy is a life-saving drug in opening a physiologically closed ductus and maintaining its patency, though with several side effects. PGE1 can cause peripheral vasodilation and hypotension, necessitating the securement of two peripheral intravenous lines, one for prostaglandin infusion and the other for giving maintenance fluid and drugs. It can also cause neurological side-effects such as jitteriness, seizure-like activities, respiratory depression, and apnea, which can be potentiated by sedatives used during the procedure, especially in preterm and low birth weight babies. Respiratory depression was reported in 12% of neonates by Lewis et al.,[9] though it did not specify its intraprocedural rate. Twelve (70 %) of patients were on PGE1 infusion in our study. Cirstovenu et al.,[6] have all their 25 patient of BAS on PGE1 infusion. Al-Kassmy et al., reported administration in 124 out of 134, accounting for 92.5% of patients.[4] This represents widespread, almost universal use of PGE1 in TGA. Our study did not show any case of respiratory depression during the procedure. Respiratory monitoring is of utmost monitoring to prevent hypoventilation and hypoxia, especially in spontaneously breathing patients on IV sedation.
The mode of anesthesia to be used is governed by the cardiorespiratory status of the patient. Usually, severely cyanotic, acidotic patients on inotropic supports are already intubated before coming to the catheterization laboratory. In our study, patients, injections of ketamine 0.5–1 mg/kg and glycopyrrolate ten μg/kg were given for intravenous anesthesia. They were repeated every 30 min or on patient movement along with oxygen supplementation with face mask or nasal prongs at 4 L/min. General anesthesia was induced with an injection ketamine 2 mg/kg, injection glycopyrrolate 10 μg/kg, fentanyl 2 μg/kg, muscle relaxant cis-atracurim 0.1 mg/kg, or vecuronium 0.08 mg/kg. For maintenance, sevoflurane with oxygen + air mixture and boluses of fentanyl 0.5 μg/kg with vecuronium/cisatracurium boluses were administered. All patients were monitored with standard (American Society of Anesthesiologist) monitors. Patients intubated before their arrival in the catheterization laboratory were continued with general anesthesia and mechanical ventilation; all other patients were given intravenous anesthesia with monitored anesthesia care. In our study, 6 (35%) patients received intravenous sedation versus 11 (65%) patients received general anesthesia with endotracheal intubation. General anesthesia was used in 31.3% of patients, whereas conscious sedation was used in 68.7% of patients in a retrospective analysis of 192 patients by Matter et al.,[2] showing more patients receiving sedation. Irrespective of the mode of anesthesia used in accordance to institutional protocols and patient’s condition, maintaining hemodynamic stability, avoiding hypothermia in a cold catheterization laboratory environment, and being prepared for encountering both anticipated (facial dysmorphism associated with chromosomal anomalies and congenital heart disease) and unanticipated difficult airway scenarios form the goals of anesthesia management.[10]
Complications encountered during atrial septostomy could be classified into mechanical, traumatic, embolical, and electrical. Complication rates have been reported in up to 11% of procedures in some series.[11] Cirstovenu et al., reported an overall 34% complication rate, most being transient.[6] Our study had a similar rate of 41%. Al-Kassmy et al. had a lower complication rate of 5.9% in 134 patients, with 3 (2.2%) being severe.[4] Maerozy encountered complications in 2 (4.17%) out of 56 patients.[5] BAS in an emergency setting and out of usual working hours has been shown to be associated with an increase in a complication rate of 47% and a mortality rate of 3% by Vimalesvaran et al.,[12]. This is in conjunction with our findings since all our procedures were done on an emergency basis with a comparatively higher complication rate.
Electrical complications are frequent and include transitory rhythm disturbances- premature ectopic beats, supraventricular tachycardia (SVT), atrial flutter and fibrillation, partial or complete heart block, and ventricular arrhythmias.[11] Our study had transient atrial arrhythmias as the most common complication in 23%, a lower incidence was found by Cirstovenu et al., of about 9.3%.[7] Premature ectopic beats in 54% of cases were found by Matter et al. followed by SVT in 8%.[2] Modalities for prompt treatment are a must in the catheterization laboratory for their treatment.
Traumatic complications include rupture of the atrial appendage, mitral valve injury, or vascular injury of the pulmonary veins or the inferior caval vein. One case of cardiac perforation of the left atrial appendage was reported by Matter et al., concurrent with our finding and was managed surgically.[2] They also found that balloon rupture occurred in 7% of cases with no embolization of balloon fragments. The finding was similar to our study of 5% each incidence of balloon rupture and pericardial effusion due to the left atrial puncture.
Stroke, as an embolic complication, was linked with the performance of BAS in some studies. There are conflicting reports of increased incidence of stroke in neonates post-BAS. Hamzah et al., in a large series of 17,392 neonates, reported stroke in 1.1% compared to 0.6% of non-BAS neonates.[13] In a recent meta-analysis by Polito et al., in 10,108 patients, which included 22.4% with BAS, they did not observe increased odds of brain injury.[14] Petit et al., in their 26 neonate study, did not find a correlation between BAS and stroke risk and, in turn, found oxygenation and time to surgery to be correlated with this adverse outcome.[15] There was no case of stroke in our study.
There were no procedural deaths in our study.
Limitations
The present study has inherent limitations besides being retrospective in design. This is a single-center study, which limits the generalizability of the conclusions as opposed to multi-centered collaborative studies or prospective protocol-based studies. Second, the sample size being small also limits the generalization of results compared to a large study group.
CONCLUSION
BAS is a safe and effective palliative procedure for TGAIVS, with good immediate results in our institution. Urgent BAS is often the only solution to restore adequate systemic perfusion of oxygenated blood in TGA with IVS. Maintaining cardiorespiratory stability, prevention of respiratory depression in a spontaneously breathing neonate, and preparation for emergency intubation is always warranted for any untoward complication, maintenance of normothermia in the cold temperature of a catheterization laboratory with active warming means, and eternal vigilance forms the cornerstone of successful neonatal outcome.
Ethical approval
The research/study complied with the Helsinki Declaration of 1964.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Minati Choudhury is the member of the editorial board of the journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Perioperative Physiology, Imaging and Management of Transposition of Great Arteries. Semin Cardiothorac Vasc Anaesth. 2015;19:210-22.
- [CrossRef] [PubMed] [Google Scholar]
- Balloon Atrial Septostomy: The Oldest Cardiac Interventional Procedure in Mansoura. Egypt Heart J. 2011;63:125-9.
- [CrossRef] [Google Scholar]
- Impact of Balloon Atrial Septostomy Guided by Echocardiography in Neonates with Transposition of the Great Arteries. Arch Cardiovasc Dis Suppl. 2016;8:269.
- [CrossRef] [Google Scholar]
- Balloon Atrial Septostomy: Does the Balloon Size Matter? CJC Pediatr Congenit Heart Dis. 2022;1:253-9.
- [CrossRef] [PubMed] [Google Scholar]
- The Effectiveness of Balloon Atrial Septostomy in Neonates with Great Artery Transposition in Duhok, Iraq. J Pharm Negat Results. 2022;13:389-92.
- [CrossRef] [Google Scholar]
- Impact of Bedside Ballon Atrial Septostomy in Neonates with Transposition of Great Arteries in a Neonatal Intensive Care Unit in Romania. Life (Basel). 2023;13:997.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality in Potential Arterial Switch Candidates with Transposition of the Great Arteries. J Am Coll Cardiol. 1998;32:753-7.
- [CrossRef] [PubMed] [Google Scholar]
- Fetal Predictors of Urgent Balloon Atrial Septostomy in Neonates with Complete Transposition. J Am Soc Echocardiogr. 2011;24:425-30.
- [CrossRef] [PubMed] [Google Scholar]
- Side Effects of Therapy with Prostaglandin E1 in Infants with Critical Congenital Heart Disease. Circulation. 1981;64:893-8.
- [CrossRef] [PubMed] [Google Scholar]
- Anaesthesia for Paediatric Diagnostic and Interventional Cardiological Procedures. Continuing Educ Anaesth Crit Care Pain. 2015;15:1-6.
- [CrossRef] [Google Scholar]
- Balloon Atrial Septostomy: History and Technique. Images Paediatr Cardiol. 2006;8:8-14.
- [Google Scholar]
- Balloon Atrial Septostomy Performed “Out of Hours”: Effects on the Outcome. Cardiol Young. 2013;23:61-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and Outcomes of Balloon Atrial Septostomy in Neonates with Transposition of Great Arteries. Pediatr Crit Care Med. 2020;21:324-31.
- [CrossRef] [PubMed] [Google Scholar]
- Balloon Atrial Septostomy and Pre-Operative Brain Injury in Neonates with Transposition of the Great Arteries: A Systematic Review and a Meta-analysis. Cardiol Young. 2012;22:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative Brain Injury in Transposition of Great Arteries is Associated with Oxygenation and Time to Surgery, not Balloon Atrial Septostomy. Circulation. 2009;119:709-16.
- [CrossRef] [PubMed] [Google Scholar]






