Translate this page into:
Eroding Pseudoaneurysm of Ascending Aorta
*Corresponding author: Chaitanya Chittimuri, Department of Cardiothoracic and Vascular Surgery, The Mission Hospital, Durgapur, West Bengal, India. chaitanya.chittimuri@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chittimuri C, Javali S, Shetty V, Jasapara A, Shinde PD. Eroding Pseudoaneurysm of Ascending Aorta. J Card Crit Care TSS. 2025;9:66-70. doi: 10.25259/JCCC_37_2024
Abstract
Aortic pseudoaneurysm after cardiac surgery is a very rare and life-threatening scenario, especially when it erodes bony structures in the chest. The foremost challenging aspect is to choose the safest approach for sternotomy. Herein, the authors report a case of a 22-year-old woman with a giant pseudoaneurysm of the ascending aorta, which was eroding through the sternum. Six years prior, she underwent ventricular septal defect repair through midline sternotomy. The giant pseudoaneurysm arose from the anterior aspect of the aorta, extended to the retrosternal region right hemithorax, exerted a mass effect on the main and right pulmonary arteries, and further eroded the bony structures of the sternum. Although the sternotomy was uneventful, the surgical team had to face an inadvertently ruptured pseudoaneurysm. The authors discuss the characteristics of this case, factors that guide surgical management options, and safe bailout measures.
Keywords
Ascending aortic pseudoaneurysm
Bony erosion
Ruptured aneurysm
Circulatory arrest
INTRODUCTION
Aortic pseudoaneurysm after cardiac surgery is rare and dangerous, especially when it erodes through the sternum. The main challenge is choosing the safest way to perform sternotomy. This case report describes a large pseudoaneurysm of the ascending aorta, which had eroded through her sternum as a late sequel following a cardiac surgery in the past. The pseudoaneurysm extended into the chest and right side, pressing on the main and right pulmonary arteries, and further eroding the sternum. Although the sternotomy went smoothly, the surgical team faced an unexpected rupture of the pseudoaneurysm. This report discusses the case details, factors influencing surgical decisions, and safe emergency measures.
CASE REPORT
A 22-year-old young woman presented with a complaint of pulsatile swelling on the front of her chest, which was noticed about 4 months ago and is progressively increasing in size. The increase in size of the swelling was associated with worsening dyspnea and chest pain. No chest trauma, fever, or headache was reported by the patient.
She underwent ventricular septal defect repair 6 years back, following which she required 3 weeks of hospital stay in view of upper sternal wound infection and was doing well after discharge till she noticed the swelling 4 months back. The palpable pulsating mass in the upper part of the chest was recently noticed to be increasing in size more rapidly in the past 2 weeks before she sought medical help.
On admission to the hospital, the 1.56 m tall patient, weighing 44 kg, had a blood pressure of 118/56 mmHg, a heart rate of 72 beats/min, oxygen saturation of 96% on room air, respiratory rate of 14 breaths/min, and temperature of 37.2°C. She was cooperative, following commands well, alert, and in moderate respiratory distress. On auscultation, a regular heart rhythm with a bruit was heard in the aortic area and diffuse crackles bilaterally. The peripheral pulses were palpable well, and mild pedal edema was noted up to the lower third of the leg on both sides.
Chest roentgenogram [Figure 1] and computer tomography [Figures 2 and 3] scan revealed a large mass (pseudoaneurysm) 12 cm × 10 cm × 10 cm in size with thrombus along all walls except the mouth, which was arising from ascending aorta 2 cm above the level of aortic valve. The mass was extended into the right hemithorax, posteriorly compressing the main and right pulmonary artery, and anteriorly protruded out of the chest wall, eroding through the sternal bone, and covered only by a thin strip of soft tissue and skin.
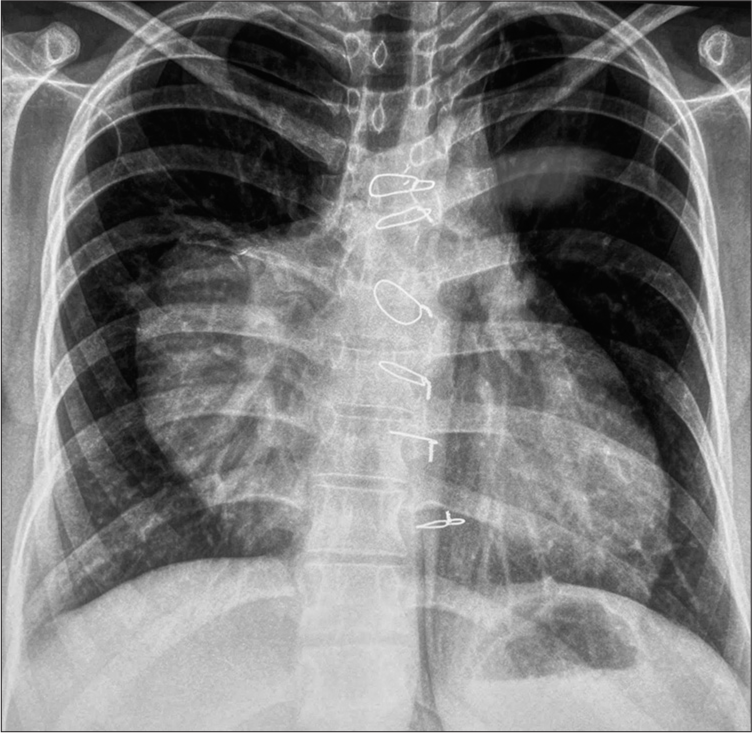
- Chest roentgenogram in PA view showing the extent of pseudoaneurysm.
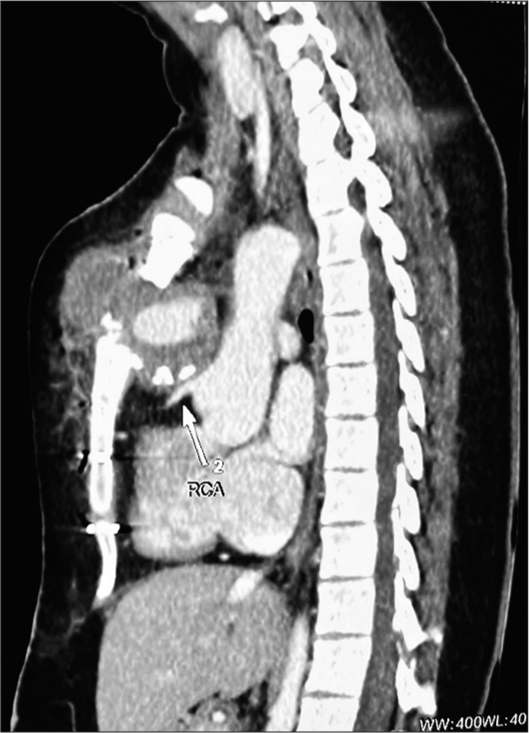
- Computer tomography scan of the patient demonstrating a pseudoaneurysm of the ascending aorta, its relation with other mediastinal structures, and erosion through a portion of the sternum. The white arrow pointing towards the Right Coronary Artery (RCA) and its proximity to sternum can be noted.
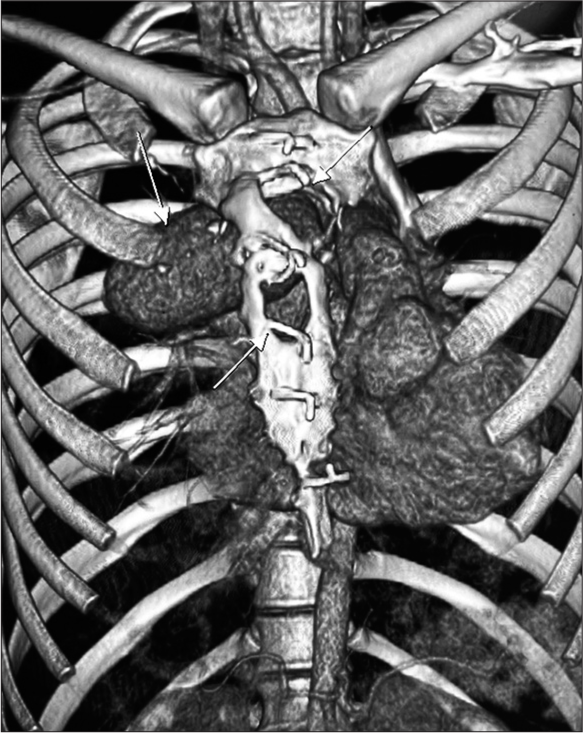
- 3D rendered reconstruction image showing the defect in sternum due erosion by the pseudoaneurysm arising from ascending aorta. The white arrows point the bony defects due to erosion by the growth of psuedoaneurysm
After informed consent, the patient was prepared for redo sternotomy, repair of pseudoaneurysm, and sternal reconstruction with pectoralis major myocutaneous (PMMC) flap. The difficulty in redoing sternotomy, the high risk of massive hemorrhage due to the large-sized pseudoaneurysm, its anterior location, and erosion out of the sternum was anticipated. An 8 mm graft was sutured to the right axillary artery, and the graft was cannulated with a 22 Fr arterial cannula. The right femoral vein was cannulated with a 25 Fr venous cannula, and cardiopulmonary bypass (CPB) was commenced before attempting sternotomy. Temperature was kept at 37°C. Redo sternotomy was done using an oscillatory saw. The upper manubrium and lower sternum were divided, and the pseudoaneurysm was dissected to open the sternum. Gentle attempts to dissect the superior vena cava, right atrium, and right ventricle (RV) to access the right superior pulmonary vein (RSPV) to vent the left ventricle (LV) failed due to dense adhesions. The surgical team proceeded with dissecting around the ascending aorta to get space to pass the cross-clamp above the pseudoaneurysm. During these maneuvers, the pseudoaneurysm ruptured. Mean perfusion pressures were maintained with great difficulty using suckers to clear the pericardial surgical field. Attempts to close the aortic rent with fingers or clamps failed to friable aortic wall. 14 Fr Foleys’ catheter was gently introduced through the rent, and the balloon was inflated to get control of bleeding. Further cooling was started to achieve a total circulatory arrest. As the temperature decreased to 32°, the heart started to fibrillate. Multiple attempts to deliver shocks with an external defibrillator and pacing with an RV pacing wire failed. On transesophageal echocardiography (TEE), LV looked non-distended but had stasis as evident by spontaneous echocardiographic contrast on TEE. The surgeon had identified that the balloon of Foley’s catheter was obstructing the whole outflow of the ascending aorta and decided to deliver a full dose of Del-Nido cold blood antegrade cardioplegia through Foley’s catheter as the balloon itself was acting like ascending aortic cross-clamp [Figure 4]. The heart was arrested in diastole, and total circulatory arrest (TCA) was achieved by further cooling to 18°C. On TCA, the Foley’s catheter was removed, and the defect in the ascending aorta, coronary ostia, aortic valve, and the rest of the visible ascending aortic anatomy was examined. Thrombus, debris, and unhealthy margin of the aortic wall were removed, and the bovine pericardial patch was used to close the ascending aortic defect in TCA with a duration of 13 min. A root vent cannula was inserted in the distal ascending aorta for venting and circulation restarted. The patient was warmed slowly and weaned off successfully and serial decannulation was done. The operating team was successful in approximating the sternum together with steel wires over drains, and the defects in the sternum were closed with muscle and subcutaneous tissues closed in layers. In view of relatively smaller defects in bone, which the team was able to satisfactorily address by closing the overlying soft tissues, the need for a PMMC flap was ruled out. She was shifted to the intensive care unit in stable condition and was extubated the next day morning. The patient was discharged home on post-operative day 7 and was doing well in subsequent visits.
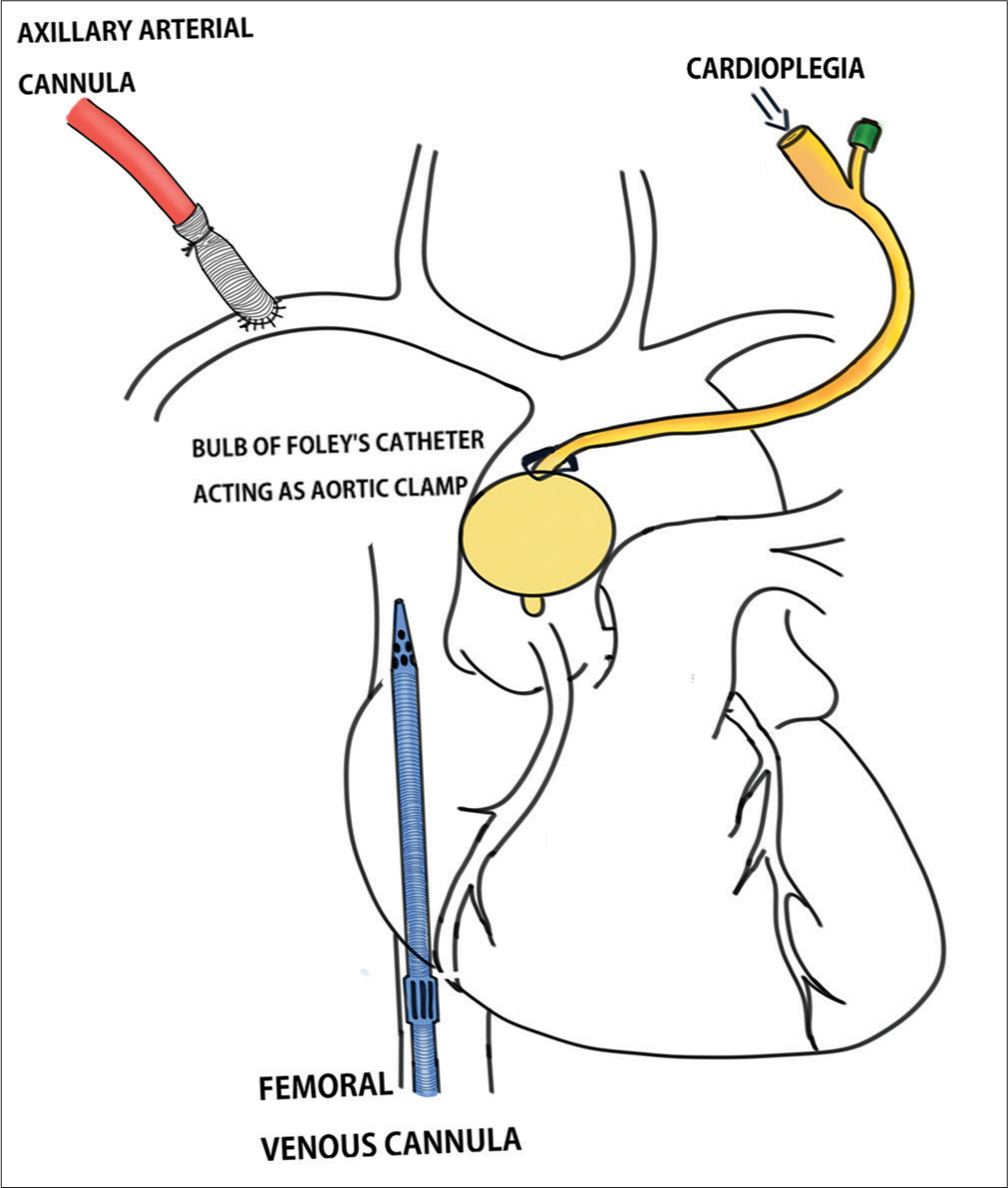
- Schematic diagram depicting cannulation strategy and cardioplegia delivery.
DISCUSSION
Ascending aortic pseudoaneurysms (AAPs) are rare, life-threatening sequelae of cardiac surgery.[1] These can also occur as sequelae to infection, genetic disorders, or trauma.[2]
Among the post-operative cases, infection, pathologic conditions of the aortic wall, dissection of the native aorta, and excessive use of biological glue are recognizable causative factors for the development of AAP.[3] These aneurysms are contained by the adventitia and the surrounding structures of the mediastinum, while the actual disruption in continuity is limited to layers of the intima and media of the Aorta. The reported incidence of AAPs has been as low as 0.5%; however, in patients with a history of cardiac or aortic surgery, higher incidence rates (up to 13%) were reported.[2] The AAPs are challenging cases for surgeons due to their high morbidity and mortality rates.[1,4]
In this age of endovascular techniques, despite advances, the treatment of AAPs is chiefly surgical.[3] The use of percutaneous stent-graft placement, device occluder implantation, and coil embolization have been reported in the treatment of AAPs. These techniques have limitations, depending on the location, size of the opening, and availability of adequate landing zones.[5] Recent literature is suggestive of improved results in surgical management owing to better investigating modalities and pre-operative planning.[3]
The progressive growth of an AAP can occur slowly over time unless intervened and may erode the bony structures of the sternum. Rupture is most dreaded and imminent in AAPs of this kind. During the progression of the disease, patients can be asymptomatic in the early stages and turn symptomatic later. In symptomatic patients, reoperation should not be delayed, and all asymptomatic patients should be informed of the possible life-threatening evolution and kept under close watch.[3]
Our patient described had a large AAP with an anterior location right underneath the sternum with sternal erosion, all forming the perfect recipe for rupture of AAP anytime. Adding further, the mass effect of the aneurysm compromised the normal functioning of the circulatory and respiratory systems, as evident by his symptoms. These led us to make a surgical plan on an urgent basis. The mass effect on surrounding structures, when swelling is more posterior, may present with dysphagia, stridor, and hoarseness.[4]
The major and prime most challenge was the approach to reentering the chest with the danger of aneurysm rupture and catastrophic hemorrhage or cerebral air embolism in every step of the procedure.[1] The patients with anteriorly located AAPs situated <2 cm from the sternum are considered high-risk patients for chest re-entry[2], and the patient being described falls into this category. Many authors, as seen through a literature search have advocated the institution of CPB before sternotomy.[6]
The extracorporeal circulation and cerebral perfusion strategies and cannulation techniques are widely varied. Femoral, carotid, and axillary artery cannulations are the most used techniques in such cases before going on to CPB.[7,8]
The authors preferred axillary cannulation due to the ease and familiarity of our team with the technique, the possibility of good antegrade flow across all limbs, viscera, and brain, low embolic risk, no need for distal perfusion cannula if needed to stay on CPB for longer periods, and being vessel which is least involved by atherosclerosis or dissection. The team avoided carotid artery cannulation because of the risk of stroke.[1] The best approach to CBP is still debatable and depends upon the site and size of the AAP and the expertise of the operating team.
The next challenge on priority is dealing with the rupture of AAP. Obtaining control of the aorta, minimizing exsanguination, maintaining good mean perfusion pressures, and protecting the brain while avoiding distension of LV should be goals of whatever method is used to deal with this scenario. The authors used a Foley’s catheter to control the bleeding, have a clear field, and arrest the heart. Although this was not expected, the sheer presence of mind led the team to use this technique. Apaydin et al. reported a similar use of Foley’s catheter to control bleeding when the aneurysm was injured during sternotomy.[9] An identical idea with the alternative route of the balloon occlusion technique was documented by Pettersson et al.[10] using an intraaortic occlusion balloon catheter through a transfemoral route to minimize the risk of rupture of an aortic wall defect. Although both techniques effectively dealt with the bleeding, they did not minimize ventricular distention. The authors advocate the use of a left atrial vent through RSPV when possible before meddling with AAP. Few authors have suggested the use of a left atrial vent through a left mini-thoracotomy when redo sternotomy preludes dissection from the anterior.[11] When the above is not possible, the LV can be vented through the apex, depending on the ease of access.[12]
While we, as many, opted to go on for TCA when there is an inadvertent rupture of aneurysm, low-flow hypothermic perfusion with or without circulatory arrest is a possibility in cases with unruptured AAPs.[12] The use of port access is reported, which permits a solution, permitting endo-aortic cross clamping, LV venting, and cardioplegia administration before incision and circulatory arrest. This approach may avoid complete dissection of the aorta for adequate aortic cross-clamping and myocardial protection. The feasibility of this technique may vary from patient to patient and center to center.[13]
Pseudoaneurysm repair techniques range from isolated direct closure of the lesion’s neck and patch closure of the defect to extensive repair with aortic-graft replacement and should be chosen wisely and catered according to pathology and anatomy in each patient.
CONCLUSION
Meticulous planning, involving surgeons, anesthetists, perfusionists, nurses, and assistants, was conducted. Prompt decision-making, smooth execution, and a bit of luck contributed to a good result being achieved by the authors. The appropriate cannulation strategy and use of hypothermic circulatory arrest enable the dissection of the mediastinum and control of AAP. Anticipating things that could go wrong in each step of the procedure preparing the team to tackle each of them with the best possible bailout plans should make teams tackle such life-threatening scenarios efficiently.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Surgical Management of a Giant Ascending Aortic Pseudoaneurysm. Tex Hear Inst J. 2010;37:710-3.
- [Google Scholar]
- Systematic Review of Interventions to Repair Ascending Aortic Pseudoaneurysms. Ochsner J. 2014;14:576-85.
- [Google Scholar]
- Analysis of Postsurgical Aortic False Aneurysm in 27 Patients. Tex Hear Inst J. 2013;40:274-80.
- [Google Scholar]
- Pseudoaneurysms of the Ascending Aorta Following Coronary Artery Bypass Surgery. J Card Surg. 2006;21:221-4.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous Closure of Aortic Pseudoaneurysm by Amplatzer Occluder Device-Case Series of Six Patients. Catheter Cardiovasc Interv. 2009;73:521-9.
- [CrossRef] [PubMed] [Google Scholar]
- Reoperation for Giant False Aneurysm of the Thoracic Aorta: How to Reenter the Chest? Ann Thorac Surg. 2007;83:1610-4.
- [CrossRef] [PubMed] [Google Scholar]
- Unusual Case of Pseudoaneurysm of Ascending Aorta Eroding Presternal Soft Tissue and Sternum in a Patient with Miliary Tuberculosis and A Bioprosthetic Aortic Valve. J Clin Images Med Case Rep. 2021;2:1523.
- [CrossRef] [Google Scholar]
- Pseudoaneurysm of Ascending Aorta after Aortic Valve Replacement. Ann Thorac Surg. 2005;79:705-7.
- [CrossRef] [PubMed] [Google Scholar]
- A Practical Tool to Control Bleeding During Sternal Reentry for Pseudoaneurysm of the Ascending Aorta. Ann Thorac Surg. 2003;75:1037-8.
- [CrossRef] [PubMed] [Google Scholar]
- Transfemoral Control of Ruptured Aortic Pseudoaneurysm at Aortic Root Reoperation. Ann Thorac Surg. 2004;77:311-2.
- [CrossRef] [PubMed] [Google Scholar]
- Repair of Ascending Aortic Pseudoaneurysm Eroding through the Sternum. Asian Cardiovasc Thorac Ann. 2019;27:36-8.
- [CrossRef] [PubMed] [Google Scholar]
- Mediastinal False Aneurysm after Thoracic Aortic Surgery. Ann Thorac Surg. 2000;70:547-52.
- [CrossRef] [PubMed] [Google Scholar]
- Management of Pseudoaneurysm of the Ascending Aorta Performed Under Circulatory Arrest by Port-access. Ann Thorac Surg. 2001;71:1010-1.
- [CrossRef] [PubMed] [Google Scholar]






