Translate this page into:
Value of Point-of-Care Algorithms in Pediatric Cardiac Surgery
*Corresponding author: Klaus Görlinger, Department of Anaesthesiology and Intensive Care Medicine, University Hospital Essen, Essen, Germany. kgoerlinger@werfen.com
-
Received: ,
Accepted: ,
How to cite this article: Görlinger K, Kammerer T. Value of Point-of-Care Algorithms in Pediatric Cardiac Surgery. J Card Crit Care TSS. 2024;8:181-4. doi: 10.25259/JCCC_43_2024
The use of point-of-care (POC) viscoelastic testing-guided bleeding management algorithms is well established in patient blood management (PBM) guidelines for adult cardiac and non-cardiac surgery with a grade 1B-R to 1A recommendation.[1,2] Here, meta-analyses of randomized controlled trials (RCTs) demonstrated a significant reduction in transfusion requirements, morbidity (e.g., acute renal failure), and mortality.[3] However, the evidence of viscoelastic testing-guided bleeding management algorithms for pediatric cardiac surgery is still low due to a limited number of studies, particularly RCTs. Up to now, two small RCTs assessed the effect of developing and implementing thromboelastometry-guided bleeding management algorithms and reported a significant reduction in post-operative bleeding, transfusion, duration of mechanical ventilation, and length of stay at the intensive care unit (ICU) but no change in in-hospital mortality.[4,5] These results have been confirmed by recent cohort studies in neonatal and infant cardiac surgery.[6] In this issue of the Journal of Cardiac Critical Care TTS, Chakraborty et al. demonstrated not only a significant reduction in chest tube drainage, transfusion requirements (packed red blood cells [PRBCs], plasma, and platelets; P < 0.001, each), postoperative complication rates (risk ratio [RR] {95% confidence interval [CI]}; 0.6467 [0.4763–0.8782]; P = 0.0052), and length of stay at the ICU but also a strong trend for reduced in-hospital mortality (RR [95% CI]; 0.4375 [0.1571–1.2187]; P = 0.1138).[7] The authors can be congratulated for their great study showing that the implementation of POC-guided bleeding management in pediatric cardiac surgery cannot only reduce bleeding and transfusion but also improve patient outcomes regarding post-operative complication rates and mortality, as shown for adult cardiac surgery.[3] Accordingly, all efforts should be made to improve patient outcomes and save the lives of our youngest patients undergoing cardiac surgery.
How can this be achieved? First of all, restrictive transfusion thresholds for all blood components must be implemented for pediatric cardiac surgery. Deng et al. reported in their systematic review and meta-analysis on packed red blood cell (PRBC) transfusion thresholds after pediatric cardiac surgery that a restrictive transfusion strategy with a hemoglobin (Hb) threshold of 7–9 g/dL is at least non-inferior to a liberal Hb threshold of 9.5–13 g/dL (RR [95% CI]; 0.49 [0.13–1.94]; P = 0.31).[8] However, a restrictive transfusion strategy must be implemented for the yellow blood products plasma and platelets, too, since they contribute significantly to transfusion-associated morbidity (transfusion-associated circulatory overload, transfusion-related lung injury, and transfusion-related immunomodulation with nosocomial infections, organ failure and sepsis and mortality).[9] Particularly, massive bleeding (blood loss of more than one total blood volume within 24 h) and transfusion (transfusion of more than 40 mL PRBCs within 24 h) should be avoided since it is associated with much higher mortality in pediatric patients compared to adults.[10]
However, PBM goes beyond restrictive blood transfusion and aims to improve patient outcomes by managing and preserving the patient’s own blood while promoting patient safety. Here, minimizing surgical, procedural, and iatrogenic blood losses and managing coagulopathic bleeding are cornerstones of the second pillar of PBM. To achieve this, the development and implementation of patient-centered, evidence-based POC thromboelastometry-guided bleeding management algorithms are important to facilitate personalized precision medicine in perioperative bleeding management as good medical practice. Here, the goal should be to administer the right hemostatic drug or intervention to the right patient at the right time, in the right dose, and in the right sequence while limiting unnecessary exposure to improve patient safety and outcomes as part of a comprehensive pediatric PBM strategy.[9,11,12]
Both with and without the use of the POC technique, PBM entails important considerations as outlined in Tables 1 and 2. Judicious Rotational thromboelastometry (ROTEM) or Thromboelastography (TEG) use can overcome many bleeding situations, as seen in Table 2 and Figure 1.[11,12]
|
rFVIIa: Recombinant activated factor VII, TACO: Transfusionassociated circulatory overload, TRALI: Transfusionrelated lung injury, TRIM: Transfusionrelatedimmunomodulation, CPB: Cardiopulmonary bypass, POC: Pointofcare, Hb: Hemoglobin, INR: International normalized ratio, ROTEM/TEG: Rotational thromboelastometry / Thrombelastography; FIBTEM: Fibrin-based thromboelastometric test, APTEM: Activated Partial thromboelastometric test, HEPTEM: Heparinase assay based thromboelastometric test, EXTEM: Extrinsically activated thromboelastometric test, PRBC: Packed red blood cells, APTEM: Aprotinin based thromboelastometric test for hyperfibrinolysis
| S. No. | Goal | ROTEM delta or sigma | or | TEG 5000 or TEG 6s | Supplement with |
|---|---|---|---|---|---|
| 1. | Rule out residual heparin (and avoid Protamine overdose) | ACT postprotamine >1.2×ACT baseline and INTEM CT/HEPTEM CTratio ≥1.25 |
OR | ACT postprotamine >1.2×ACT baseline AND KaolinTEG Rtime >heparinaseTEG Rtime×1.25 |
Protamine (0.3–0.5 mg/kg) |
| 2. | Restore fibrinogen | EXTEM A5 <25 mm AND FIBTEM A5 <7 mm (FIBTEMbased dose calculation) | OR | RapidTEG or kaolin TEG MA <40 mm AND TEG FF <10 mm |
Fibrinogen concentrate OR cryoprecipitate |
| 3. | Restore platelets (additional platelet function testing may be considered) | EXTEM A5 <25 mm AND FIBTEM A5 ≥7 mm |
OR | RapidTEG or kaolinTEG MA <40 mm AND TEG FF ≥10 mm |
Platelets (5–10 mL/kg) ±DDAVP (0.3 μg/kg) |
| 4. | Replace enzymatic factors (thrombin generation) | EXTEM CT>90 s AND FIBTEM A5≥7 mm (since low fibrinogen prolongs CT results but cannot be treated with FFP or 4FPCC) |
OR | Heparinase TEG Rtime >12 min (Notably, TEG Rtime cannot be used to guide PCC since this requires an extrinsically activated assay) |
FFP (10–15 mL/kg) OR 4FPCC (10–15 U/kg) |
| 5. | Block fibrinolysis (Treat as early as suspected/detected) | EXTEM A5 <25 mm OR EXTEM or FIBTEM ML >7% @ 30 min (LI30 <93%) OR EXTEM or FIBTEM ML >15% @ 60 min (LI60 <85%) | OR | LY30 >7.5% (Notably, TEG LY30 is 30 min after MA and ROTEM LI30 is 30 min after CT) | EACA or TXA |
POC: Pointofcare, TXA: Tranexamic acid, EACA: Epsilonaminocaproic acid, ACT: Activated clot time, DDAVP: Desmopressin acetate, FFP: Fresh frozen plasma, PCC: Prothrombin complex concentrate, EACA: Epsilon-aminocaproic acid, TXA: Tranexamic acid, ROTEM: Rotational thromboelastometry
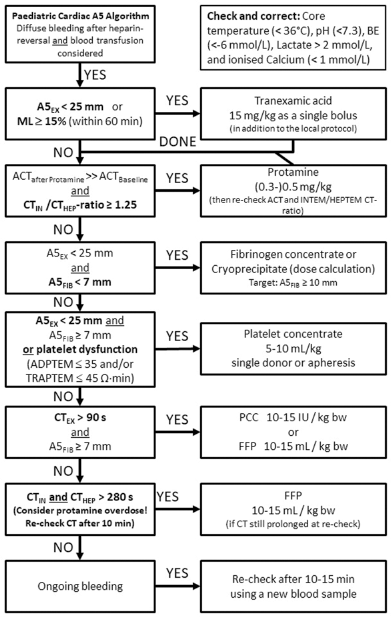
- Evidence-based thromboelastometry (ROTEM)-guided bleeding management algorithm for pediatric cardiac surgery (Courtesy of Klaus Görlinger, Essen, Germany). ML: Maximum lysis, FFP: Fresh frozen plasma, PCC: Prothrombin complex concentrate, ACT: Activated clot time, TRAPTEM: ROTEM platelet assay with Thrombin receptor-activating peptide, HEPTEM: Heparin based thromboelastometric test for hyperfibrinolysis. ADPTEM: Adenosine di-phosphate based thromboelastometric test.
In summary, the implementation of thromboelastometry-guided bleeding management algorithms in pediatric cardiac surgery requires education, knowledge, and interdisciplinary communication, collaboration, and consensus, but – if implemented – cannot only reduce transfusion requirements and costs but also has a high potential to improve patient safety and outcomes in this vulnerable patient population. This has been demonstrated impressively by the team from AIIMS in New Delhi, India, and I hope that others will follow their path.[7]
Conflicts of interest
KG works as the Medical Director of TEM Innovations GmbH/Werfen PBM, Munich. Germany.
References
- STS/SCA/AmSECT/SABM Update to the Clinical Practice Guidelines on Patient Blood Management. J Cardiothorac Vasc Anesth. 2021;35:2569-91.
- [CrossRef] [Google Scholar]
- 2022 ESC Guidelines on Cardiovascular Assessment and Management of Patients undergoing Non-Cardiac Surgery. Eur Heart J. 2022;43:3826-924.
- [CrossRef] [Google Scholar]
- Viscoelastic Haemostatic Assays in the Perioperative Period of Surgical Procedures: Systematic Review and Meta-Analysis. J Clin Anesth. 2020;64:109809.
- [CrossRef] [Google Scholar]
- Thromboelastometry-guided Intraoperative Haemostatic Management Reduces Bleeding and Red Cell Transfusion after Paediatric Cardiac Surgery. Br J Anaesth. 2015;114:91-102.
- [CrossRef] [Google Scholar]
- A Prospective Randomized Clinical Trial of Efficacy of Algorithm-based Point-of-Care guided Hemostatic Therapy in Cyanotic Congenital Heart Disease Surgical Patients. J Card Crit Care TSS. 2020;3:8-16.
- [CrossRef] [Google Scholar]
- A ROTEM-Guided Algorithm Aimed to Reduce Blood Product Utilization During Neonatal and Infant Cardiac Surgery. J Extra Corpor Technol. 2023;55:60-9.
- [CrossRef] [Google Scholar]
- Randomised Controlled Trial using AIIMS Simplified Point-of-Care Algorithm for Bleeding Management in Cyanotic Children undergoing Cardiac Surgery. J Card Crit Care. 2024;8
- [Google Scholar]
- Red Blood Cell Transfusion Threshold after Pediatric Cardiac Surgery: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2019;98:e14884.
- [CrossRef] [Google Scholar]
- Pediatric NonRed Cell Blood Product Transfusion Practices: What's the Evidence to Guide Transfusion of the 'Yellow' Blood Products? Curr Opin Anaesthesiol. 2020;33:259-67.
- [CrossRef] [Google Scholar]
- The Role of Evidence-based Algorithms for Rotational Thromboelastometry-guided Bleeding Management. Korean J Anesthesiol. 2019;72:297-322.
- [CrossRef] [Google Scholar]
- Utility of Platelet Function Testing in Cardiac Surgery in 2021. J Card Crit Care. 2021;5:84-7.
- [CrossRef] [Google Scholar]






