Translate this page into:
Functional Evaluation of Microcirculation in Response to Fluid Resuscitation in Hypovolemic Adult Post-cardiac Surgical Patients
*Corresponding author: P. S. Nagaraja, Department of Anesthesiology, Sri Jayadeva Institute of Cardiovascular Sciences and Research, Bengaluru, Karnataka, India. docnag10@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhavya G, Gupta A, Nagesh KS, Murthy PR, Nagaraja PS, Ragavendran S, et al. Functional evaluation of microcirculation in response to fluid rescuscitation in hypovolemic adult post-cardiac surgical patients. J Card Crit Care TSS 2023;7:48-54.
Abstract
Objectives:
Microcirculation is bound to be altered during cardiac surgery due to multiple factors, mainly the intense systemic inflammatory response syndrome which peaks in the first 24-h postoperatively. Decreased microvascular flow associated with increased postoperative morbidity has been reported. The literature suggests a potential independence of macrocirculation and microcirculation during fluid loading. The present study was conducted to assess thenar muscle tissue oxygen saturation (StO2) changes during vascular occlusion test (VOT) in response to hypovolemia and to assess the dynamic responses of the StO2 variables post-volume expansion (VE).
Material and Methods:
Thirty-five adult post-cardiac surgical patients, with stroke volume (SV) variation >12% were included in the study. Fifty-two fluid challenges were studied. Functional evaluation of microcirculation using VOT and near infrared spectroscopy (NIRS) variables along with monitoring of macrocirculatory indices was performed before and after VE. Statistical analysis was done using Student t-test.
Results:
Post-VE, 34 were responders with increase in SV ≥15% and 18 were non-responders (SV <15%). Rate of resaturation was significantly faster in responders compared to non-responders after VE (P = 0.0293 vs. P = 0.1480). However, macrocirculatory indices including cardiac output, SV, and delivery of oxygen showed significant improvement in both responders and non-responders.
Conclusion:
Preload dependence is associated with significant change in the StO2 recovery slope measured at the thenar eminence in volume responders. Functional evaluation of microcirculation using VOT and StO2 can be a useful complimentary tool along with the macrocirculatory indices for optimal fluid rescuscitaion in adult post-cardiac surgical patients.
Keywords
Fluid responsiveness
Microcirculation
Macrocirculation
Near infrared spectroscopy variables
Vascular occlusion test
Cardiac surgery
INTRODUCTION
Goal directed therapy has been used for nearly 30 years, being one of the most prevalent strategy in fluid rescuscitation which has shown to improve both short-term and long-term outcome.[1,2] Early goal directed therapy (EGDT) has been extensively studied in septic shock patients when fluid is administered within 6 h of presentation to the emergency department. Evidence supporting the use of EGDT in sepsis was found by the landmark Rivers trial, which showed a 16% of mortality reduction.[3] The literature shows that EGDT has also been beneficial when employed postoperatively after major high risk surgeries.[4,5]
Correct assessment of hypovolemia and fluid responsiveness is critically important in patients with compromised cardiac and pulmonary function. Microcirculation has been found to be altered during cardiac surgery due to multiple factors such as anesthesia, surgery, hypothermia, microemboli,[6,7] and the intense systemic inflammatory response syndrome which peaks in the first 24-h postoperatively.[8]
Fluid therapy guided by functional hemodynamic indices of volume responsiveness such as pulse pressure variation and stroke volume variation (SVV), instead of static measures such as central venous pressure, pulmonary artery pressure, or mean arterial pressure (MAP) reported a better outcome.[9] However, the previous reports have suggested a potential independence of macrocirculation and microcirculation during fluid loading.[10,11] Thus, fluid administration may correct macrocirculatory hemodynamic variables with or without improvement in microcirculation.
Decreased microvascular flow associated with increased postoperative morbidity has been reported.[12] The operative and post-operative mortality in cardiac surgery has decreased significantly in the recent years due to improved care and advanced monitoring of the macrocirculatory indices.[13-15] The post-operative morbidity is still significantly high,[13] as a result of which 10% of patients require a prolonged post-operative care.[16] The alterations in microcirculation leading to inadequate tissue oxygen delivery have been recognized as an independent marker for prolonged intensive care unit (ICU) stay.[17]
Hence, there is a need to correlate macrocirculatory variables like increase in SV with change in microcirculation, especially in critically ill cardiac surgical patients for effective fluid resuscitation.
Microcirculatory hemoglobin and oxygen availability can be measured by the use of near infrared spectroscopy (NIRS),[18] a non-invasive technique that can be performed at the bedside. Differential absorption of infrared light at two specific wavelengths (680 and 800 nm) by deoxyhemoglobin is used to define the hemoglobin saturation level in vessels located in the tissue volume that is illuminated by the probe.[19]
Tissue oxygen saturation (StO2) values were shown to have a large overlap between healthy subjects and septic shock patients which largely limits the interest of this parameter for individual care. In this respect, a vascular occlusion test (VOT) combined with StO2 measurement is proposed.[20]
The dynamic response of the StO2 recovery slope, during a standardized VOT, is assumed to reflect the recruitment of microvessels in response to a local hypoxic stimulus.[21]
In view of the limited literature to guide optimal fluid therapy in correlation with improvement in microcirculatory parameters in cardiac surgical patients, the present study was conducted to assess thenar muscle StO2 changes during VOT in response to hypovolemia and to assess the dynamic responses of the StO2 recovery slope post-volume expansion (VE).[22,23]
MATERIAL AND METHODS
Methodology
After obtaining the Institutional Ethical Committee clearance, the prospective, observational, and clinical study was performed at a tertiary care cardiac center.
Study population
Patients shifted to post-operative ICU with >18 years of age, post-elective cardiac surgery with SVV >12% were included in the study.
Patients on extracorporeal circulatory support, intracardiac shunts, left ventricular ejection fraction <35%, inotrope score of >20, emergency surgery, patients with arrhythmias, and history of peripheral vascular disease were excluded from the study.
Study protocol
In the post-operative ICU, NIRS (Equano × 7600) optode was attached to the thenar eminence and Flotrac vigileo cardiac output monitor was connected to the femoral artery. Sphygmomanometer cuff was applied around the same arm as NIRS optode.
Post-operative management was standardized for all the patients, including ventilation protocol (tidal volume of 6–8 mL/kg, positive end expiratory pressure of 5 cm of water, respiratory rate of 12–16/min). Ventilatory parameters were not altered throughout the procedure. All patients received intravenous fentanyl (1 mcg/kg) bolus before commencement of VOT.
Thenar StO2 was measured using NIRS. StO2 stability was defined as variation <2% over 30 s (pre-VOT StO2).[24]
Macrocirculatory variables were measured using Flotrac vigileo monitor.
Normal saline was used for fluid bolus.
VOT procedure
VOT involved rapidly inflating a sphygmomamoter cuff (< 3 s) until 50 mmHg above the systolic blood pressure (SBP). The cuff was released rapidly (< 2 s) after 3 min of occlusion. The StO2 occlusion slope is shown to relate to tissue oxygen consumption and the StO2 reperfusion slope is proposed to evaluate tissue flow reperfusion and vascular recruitment [Study flow diagram].[25]

Statistical analysis
Statistical analysis was performed using MedCalc version 12.2.1.0. Categorical data were analyzed with Chi-square test. Paired Student t-test was used to compare data at two different time points. Unpaired student t-test was used to compare data between two different groups. The results were tested for normality using the one-sample Kolmogorov– Smirnov goodness of fit test. Normally distributed data were presented as mean ± standard deviation. P ≤ 0.05 was considered statistically significant.
The study population was grouped into responders and nonresponders, based on the percentage increase in SV after intravascular VE. Responders were defined when an increase in SV was ≥15% to fluid challenge, Non-responders were defined when an increase in SV was <15%.
RESULTS
A total of 52 fluid challenges were studied in 35 patients who satisfied the inclusion criteria. [Table 1] summarizes the demographic profile. Study population was grouped into responders and non-responders based on the percentage increase in SV. Patients with increase in SV by ≥15% were grouped into responders and those with <15% were grouped into non-responders. [Table 2] represents the clinical parameters between the responders and non-responders which were recorded in all the patients before fluid bolus. Clinical parameters were comparable between the two groups. [Table 3] shows the changes in microcirculatory and macrocirculatory variables recorded before and after fluid bolus.
| Variable | Value |
|---|---|
| Age (yrs) | 52.06±12 |
| Sex (m/f) | 38/14 |
| Height (cm) | 163±6.5 |
| Weight (kg) | 65.6±11 |
| BMI | 24.1±3.5 |
| Comorbidities | |
| Hypertension | 26 |
| Diabetes | 10 |
| Smokers | 5 |
| Hypothyroidism | 1 |
| Euro score 2 | 0.8±0.1 |
| Type of Surgeries | |
| OPCAB | 30 |
| DVR | 2 |
| AVR | 3 |
BMI: Body mass index, OPCAB: Off pump coronary artery bypass, DVR: Double valve replacement, AVR: Aortic valve replacement, m: Male, f: female
| Variables | Responders Mean±SD | Non-responders Mean±SD | P-value |
|---|---|---|---|
| Urine output (mL/h) | 101±20 | 105±21 | 0.51 |
| SpO2 | 99.12±0.91 | 99.44±0.51 | 0.17 |
| Inotrope score | 3.11±1 | 3.3±1.7 | 0.62 |
| Vasodilator (SNP/NTG) | 1.08±0.45 | 1.2±0.2 | 0.131 |
| Temperature (nasopharyngeal) (°C) | 36.2±0.5 | 36.3±0.32 | 0.59 |
SD: Standard deviation, SNP: Sodium nitroprusside, NTG: Nitroglycerine
| Variables | Responders (n=34) | “P”-value | Non-responders (n=18) | “P”-value | ||
|---|---|---|---|---|---|---|
| Before VE | After VE | Before VE | After VE | |||
| SBP (mmHg) | 114.6±18.6 | 136.5±13.2 | < 0.0001 | 115.3±15.4 | 115.7±15.4 | 0.8427 |
| DBP (mmHg) | 66.7±13.5 | 76.2±11.0 | 0.0001 | 68.0±7.1 | 67.8±8.6 | 0.9337 |
| MAP (mmHg) | 82.5±13.3 | 96.1±10.9 | 0.0001 | 83.3±10.0 | 84.7±11.1 | 0.3335 |
| HR (beats/min) | 90.7±21.2 | 89.5±19.5 | 0.3126 | 93.2±16.3 | 92.2±17.2 | 0.1287 |
| SV (mL) | 54.3±14.8 | 73.2±17.0 | < 0.0001 | 58.6±17.8 | 63.2±18.6 | 0.0002 |
| SVV (%) | 18.1±4.3 | 14.2±4.0 | 0.0001 | 16.3±2.3 | 13.3±3.3 | <0.0001 |
| CO (L/min) | 5.0±1.2 | 6.2±1.1 | < 0.0001 | 5.2±1.4 | 5.7±1.5 | 0.0005 |
| Des StO2 (absolute) (s) | 148.2±33.6 | 162.8±21.1 | 0.0044 | 167.4±13.8 | 160.5±40.7 | 0.3874 |
| Des StO2 (%/s) | 7.7±3.0 | 7.5±3.5 | 0.5197 | 8.4±3.0 | 9.5±3.2 | 0.3499 |
| Res StO2 (absolute) (s) | 35±24.2 | 21.8±13.4 | 0.0024 | 21.8±11.2 | 27.1±14.8 | 0.0034 |
| Res StO2 (%/s) | 52.1±38.9 | 64.5±34.1 | 0.0293 | 77.5±38.6 | 67.0±27.7 | 0.1480 |
| Hyperaemia (s) | 61.0±31.05 | 46.0±20.0 | 0.028 | 46.25±12.8 | 46.00±11.9 | 0.950 |
| DO2 (mL O2/min) | 901.6±285.0 | 1101.1±280.5 | < 0.0001 | 937.9±3 13.2 | 1049.8±330.2 | 0.0020 |
| Systemic vascular resistance (dynes.sec.cm−5) | 1313±491 | 1246±309 | 0.5019 | 1231±311 | 1132±197 | 0.2654 |
| Hemoglobin (g/dL) | 12.7±2.0 | 12.5±2.1 | 0.783 | 12.7±1.9 | 12.6±1.9 | 0.861 |
| Blood lactate (mmol/L) | 2.4±1.2 | 2.1±1.1 | 0.189 | 3.3±1.6 | 3.0±1.2 | 0.499 |
| Baseline StO2 (%) | 71.8±6.8 | 73.8±9.1 | 0.317 | 79.7±10.3 | 80±10.3 | 0.934 |
Des StO2 (absolute): Time to maximum desaturation in seconds, Des StO2 (%/s): Rate of desaturation, Res StO2 (absolute): Time to resaturation till baseline in seconds, Res StO2 (%/s): Rate of resaturation, StO2: Thenar muscle saturation, VE: Volume expansion, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, MAP: Mean arterial pressure, HR: Heart rate, SV: Stroke volume, SSV: Stroke volume variation
Administration of fluid bolus resulted in significant improvement in macrocirculatory variables such as SV and cardiac output in both the groups.
In responders, the SV (mL) increased from 54.3 ± 14.8 to 73.2 ± 17.0 (P ≤ 0.0001). Similarly, cardiac output (L/min) increased from 5.0 ± 1.2 to 6.2 ± 1.1 (P ≤ 0.0001) which was statistically significant.
In non-responders, there was a statistically significant increase in SV and cardiac output after fluid bolus. Stroke volume (mL) increased from 58.6 ± 17.8 to 63.2 ± 18.6 (P = 0.0002), cardiac output (L/min) increased from 5.2 ± 1.4 to 5.7 ± 1.5 (P = 0.0005). However, the change was >15% [Figure 1].
The rate of resaturation [Graph 1a] was significantly faster after fluid administration in responders (52.1 ± 38.9–64.5 ± 34.1, P = 0.0293) compared to non-responders who did not show any significant change in the rate of resaturation (77.5 ± 38.6–67.0 ± 27.7, P = 0.1480).
However, the rate of desaturation [Graph 1b] did not show any significant change in both responders and nonresponders after administration of fluid bolus. (P = 0.5197 vs. P = 0.3499 in responders and non-responders, respectively). The time required for maximum hyperemia was significantly less in responders compared to non-responders after fluid bolus (P = 0.028 vs. P = 0.950 in responders and nonresponders, respectively).
SBP, diastolic blood pressure, and MAP demonstrated a significant increase after fluid bolus administration in responders; however, there was no significant change in non-responders.
Delivery of oxygen showed a significant increase in both the groups (P ≤ 0.0001 in responders and P = 0.0020 in nonresponders). However, the increase in DO2 was more in responders.
With regard to VE, there was a statistically significant difference in SVV in both the responders and non-responders (SVV 18.1 ± 4.3 vs. 14.2 ± 4.0, P ≤ 0.0001 and 16.3±2.3 vs. 13.3±3.3, P < 0.0001 for responders and non-responders, respectively)
Heart rate did not show any significant change after fluid administration in both responders (P = 0.3126) and nonresponders (P = 0.1287). The change in hemoglobin concentration before and after fluid bolus in both the groups was not significant as shown in [Table 3].
DISCUSSION
Adequate fluid rescuscitation remains the “holy grail” of critical care medicine. This is complicated by the fact that both under and over resuscitation is associated with increased morbidity and mortality. Therefore, the patient should receive sufficient fluid to restore adequate organ perfusion. The ideal volume replacement strategy should also improve microcirculation perfusion and tissue oxygenation.
The present study was done to assess the efficacy of fluid resuscitation in improving the microcirculation in immediate post-cardiac surgical patients. The literature reports using thenar StO2 (NIRS) have been proposed as a surrogate and non-invasive indicator of tissue oxygenation.[23,24]
In the present study, a significant improvement in resaturation rate was observed in responders in contrast to non-responders post-fluid resuscitation. These findings are in accordance with the previous study done by Tripodaki et al.[25] who assessed microcirculation with NIRS and the VOT performed at thenar eminence in 23 patients undergoing open cardiac surgery. They demonstrated that resaturation rate correlated with cardiac index and DO2 and were higher in survivors compared to non-survivors.
The previous research shows that dynamic indices are useful for predicting individualized fluid responsiveness.[26]
SVV >12% was used to predict fluid responsiveness during mechanical ventilation with a tidal volume of 8 mL/kg.[27] A similar protocol was adopted in the present study. This indicates that such patients with SVV >12% will be on the ascending portion of the Frank-Starling curve and has recruitable cardiac output.
In the present study, patients with an increase in the SV ≥15% after fluid bolus were considered as responders similar to the previous study done by Futier et al.[28] They enrolled 24 patients undergoing major abdominal surgery with evidence of hypovolemia, defined as SVV >12%. They evaluated 42 fluid challenges and found that VE leads to significant improvement in the recovery slope in both responders and non-responders without any apparent change in systemic hemodynamics. In contrast, the present study demonstrated significant faster rate of resaturation in responders (P ≤ 0.029) compared to nonresponders (P ≤ 0.148).
Payen et al.,[20] studied 43 critically ill patients with septic shock, evaluated microcirculation using VOT, a significant relationship was found between cardiac output and the reperfusion slope regardless of fluid responsiveness. They suggested that an increase in cardiac output leads to an increase in the regional perfusion and subsequent improvement of microcirculatory indices. These findings are similar to the present study as the rate of resaturation was significantly faster in responders compared to non-responders. This suggests better recruitment of microvasculature with effective fluid replacement in responders compared to non-responders who probably required further fluid administration. This finding may have important clinical implications, because the ultimate goal of resuscitation should be improvement of tissue oxygenation and perfusion.[22]
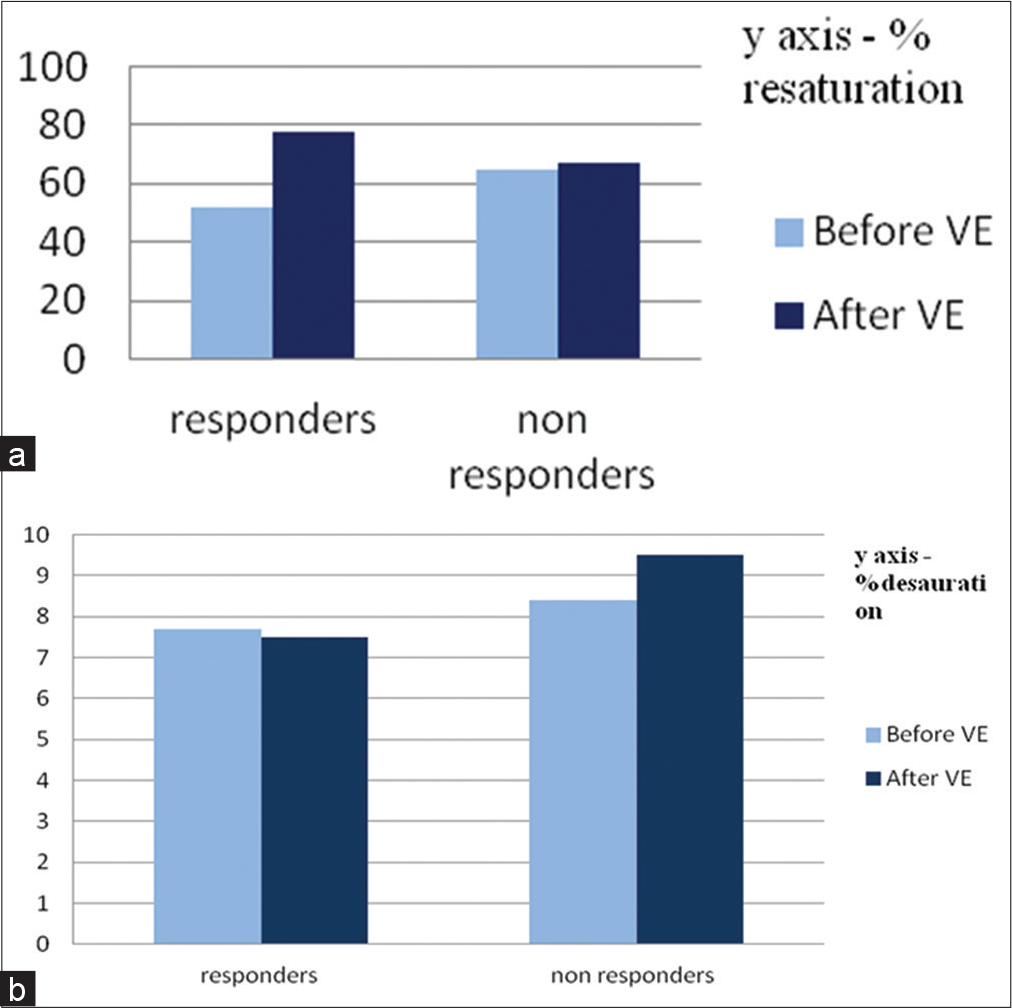
- (a) Percentage resaturation between responders and non-responders before and after volume expansion (b) Percentage desaturation between responders and non-responders before and after volume expansion.
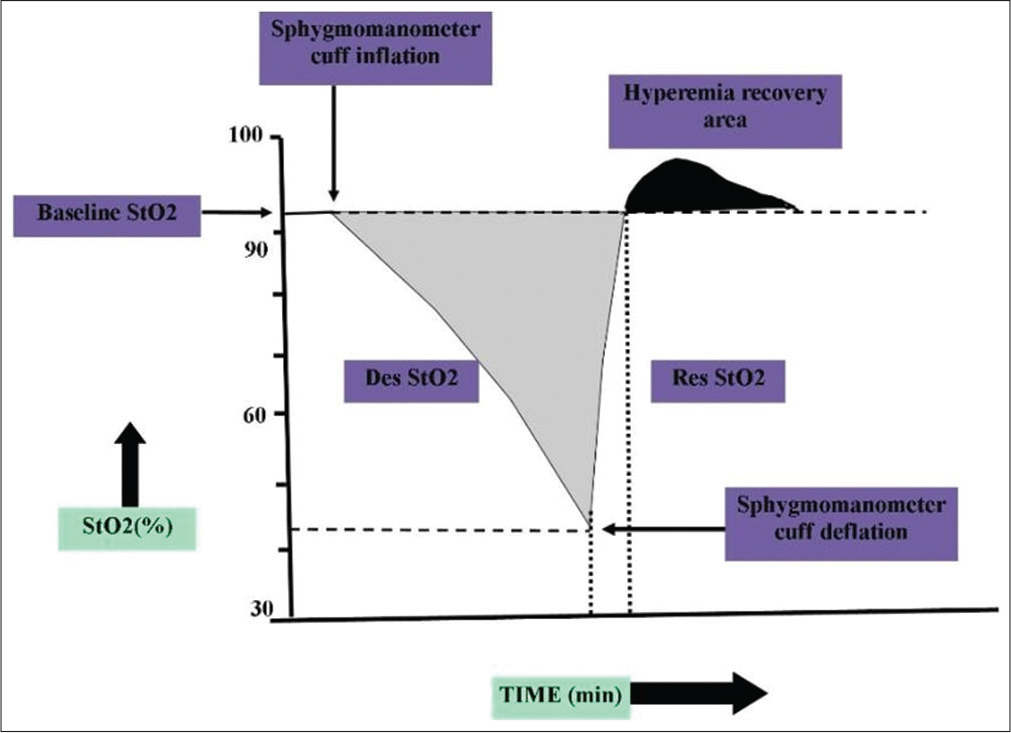
- Response to tissue oxygen saturation during a vascular occlusion test, StO2: Tissue oxygen saturation, Des StO2: StO2 desaturation slope, Res StO2: StO2 resaturation slope.
There was no significant change in the hemoglobin levels before and after VE, so it is unlikely that changes in the StO2 recovery slope with VE were due to changes in rheologic factors. This finding is in support of the study done by Creteur et al.[29] who performed VOT before and after red blood cell transfusion and found no difference despite the difference in hemoglobin levels.
It must be stressed that in non-responders, though there was a statistically significant improvement in macrocirculatory indices such as cardiac output, SV, and delivery of oxygen, the recovery slope remained statistically insignificant. This suggests that the increase in SV <15% was clinically insignificant as microcirculation and tissue oxygenation remain uncorrected.
Limitations
The present study did not determine whether faster rate of resaturation after effective fluid bolus was associated with reduced morbidity such as decreased duration of ventilation, ICU stay, and reduced organ failure. The present study is a pilot study with a small sample size.
CONCLUSION
Preload dependence is associated with significant change in the StO2 recovery slope measured at the thenar eminence in volume responders. Functional evaluation of microcirculation using VOT and StO2 can be a useful complementary tool along with the macrocirculatory indices for optimal fluid resuscitation in adult post-cardiac surgical patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112:1392-402.
- [CrossRef] [PubMed] [Google Scholar]
- Maintaining tissue perfusion in high-risk surgical patients: A systematic review of randomized clinical trials. Anesth Analg. 2011;112:1384-91.
- [CrossRef] [PubMed] [Google Scholar]
- Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-77.
- [CrossRef] [PubMed] [Google Scholar]
- Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445] Crit Care. 2005;9:R687-93.
- [Google Scholar]
- Goal-directed therapy in cardiac surgery: A systematic review and meta-analysis. Br J Anesth. 2013;110:510-7.
- [CrossRef] [PubMed] [Google Scholar]
- Microcirculatory alterations in cardiac surgery: Effects of cardiopulmonary bypass and anesthesia. Ann Thorac Surg. 2009;88:1396-403.
- [CrossRef] [PubMed] [Google Scholar]
- Monitoring of the sublingual microcirculation in cardiac surgery using orthogonal polarization spectral imaging: Preliminary results. Anesthesiology. 2007;107:939-45.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding the inflammatory response to cardiac surgery. Surgeon. 2008;6:162-71.
- [CrossRef] [PubMed] [Google Scholar]
- Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172-8.
- [CrossRef] [PubMed] [Google Scholar]
- Coupling microcirculation to systemic hemodynamics. Curr Opin Crit Care. 2010;16:250-4.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of fluid therapy on microcirculation and tissue oxygenation in hypovolemic patients: A review. Intensive Care Med. 2010;36:1299-308.
- [CrossRef] [PubMed] [Google Scholar]
- Microvascular flow and tissue oxygenation after major abdominal surgery: Association with post-operative complications. Intensive Care Med. 2009;35:671-7.
- [CrossRef] [PubMed] [Google Scholar]
- Opposite trends in coronary artery and valve surgery in a large multisurgeon practice, 1979-1999. Ann Thorac Surg. 2004;77:488-95.
- [CrossRef] [PubMed] [Google Scholar]
- A decade of change--risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990-1999: A report from the STS national database committee and the duke clinical research institute. Ann Thorac Surg. 2002;73:480-9. discussion 489-90
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and outcome following post-surgical admission to critical care. Intensive Care Med. 2011;37:1466-72.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative calculation of risk for prolonged intensive care unit stay following coronary artery bypass grafting. J Cardiothorac Surg. 2006;1:14.
- [CrossRef] [PubMed] [Google Scholar]
- Relation between oxygen consumption and oxygen delivery in patients after cardiac surgery. Anesth Analg. 1993;77:1104-10.
- [CrossRef] [PubMed] [Google Scholar]
- Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264-7.
- [CrossRef] [PubMed] [Google Scholar]
- Near infrared spectroscopy for evaluation of the trauma patient: A technology review. Resuscitation. 2006;68:27-44.
- [CrossRef] [PubMed] [Google Scholar]
- Is thenar tissue hemoglobin oxygen saturation in septic shock related to macrohemodynamic variables and outcome? Crit Care. 2009;13:S6.
- [CrossRef] [PubMed] [Google Scholar]
- Microvascular dysfunction and skeletal muscle oxygenation assessed by phase-modulation near-infrared spectroscopy in patients with septic shock. Intensive Care Med. 2005;31:1661-8.
- [CrossRef] [PubMed] [Google Scholar]
- Goal directed fluid therapy, enhanced recovery, and the perioperative surgical home for cardiac patients in noncardiac surgery In: Kaplan JA, ed. Kaplan's Cardiac Anesthesia (7th ed). Philadelphia, PA: Elsevier Publications; 2017. p. :1534.
- [Google Scholar]
- Functional hemodynamic monitoring. Curr Opin Crit Care. 2007;13:318-23.
- [CrossRef] [PubMed] [Google Scholar]
- Muscle StO2 in critically ill patients. Curr Opin Crit Care. 2008;14:361-6.
- [CrossRef] [PubMed] [Google Scholar]
- Microcirculation and macrocirculation in cardiac surgical patients. Crit Care Res Pract. 2012;2012:654381.
- [CrossRef] [PubMed] [Google Scholar]
- Automated pulse pressure and stroke volume variations from radial artery: Evaluation during major abdominal surgery. Br J Anesth. 2009;103:678-84.
- [CrossRef] [PubMed] [Google Scholar]
- Hemodynamic monitoring in the mechanically ventilated patient. Curr Opin Crit Care. 2011;17:36-42.
- [CrossRef] [PubMed] [Google Scholar]
- Use of near-infrared spectroscopy during a vascular occlusion test to assess the microcirculatory response during fluid challenge. Crit Care. 2011;15:R214.
- [CrossRef] [PubMed] [Google Scholar]
- Near-infrared spectroscopy technique to evaluate the effects of red blood cell transfusion on tissue oxygenation. Crit Care. 2009;13:S11.
- [CrossRef] [PubMed] [Google Scholar]






